Is conjugate acid always stronger than its conjugate base?
No! According to Brønsted-Lowry the strength of an acid depends on how well it can donate a proton, while the strength of a base depends on how well it can accept a proton. That is:
- strong acids are better proton donors, while weak acids are poor proton donors
- strong bases are better proton acceptors, while weak bases are poor proton acceptors
In addition to that:
- stronger acids have weaker conjugate bases, while weaker acids have stronger conjugate bases
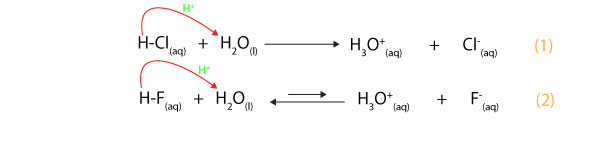
For example, let’s use the following equations to discuss the extent to which hydrochloric acid (HCl) and hydrofluoric acid (HF) will donate a proton to water:
From equation 1, you can see from the direction of the red arrow that HCl molecules donate hydrogen ions to H2O molecules. Once they donate the proton, they form chloride ion (Cl–), while H2O accepts it and forms the hydronium ion (H3O+). Because HCl molecules easily donate (ionizes) hydrogen ion to water molecules, chemists would say HCl is a strong acid (chemists usually illustrate this complete ionization of strong acid with a single arrow pointing in the forward direction). Once HCl donates a proton and turns into chloride ion (Cl–), the HCl and Cl– become conjugate acid-base pairs, where HCl is the acid and Cl–the base. However, the Cl–doesn’t easily accept a proton from water. Because of this, Cl–is a weak conjugate base of HCl. Thus, stronger acids have weaker conjugate bases.
On the other hand, from equation 2, you can see that HF molecules also donate hydrogen ions to H2O molecules. However, HF molecules are always unwilling to give up a proton to water molecules. Because of this, HF is a weak acid, while HCl is a strong acid. Chemists usually describe this incomplete ionization of HF with two arrows pointing in opposite directions, with the forward arrow relatively shorter than the reverse arrow. The shorter arrow usually stands for the fact that only fewer HF molecules donate (ionize) a proton to water molecules to generate the F– and H3O+ ions, while the longer arrow stands for the fact that the F– easily accepts a proton from H3O+ to get back HF and H2O. That is an aqueous solution of HF will contain many intact HF molecules and fewer F– and H3O+ ions. Because of this, HF and F– are conjugate acid-base pairs, where HF is a weak conjugate acid, while F– is a strong conjugate base. Thus, weaker acids have stronger conjugate bases.
To make life easier for us, we can measure the extent to which various acids donate H+ ions to water. From these measurements, we can construct a table to show the relative strengths of acids and their conjugate bases. Since we don’t have to reinvent the wheel, below is such a table constructed long time ago by chemists:

From the table, you will notice that the stronger acids are located on the top left, while stronger bases are located on the bottom right. You will also notice that weaker acids are located on the bottom left, while weaker bases are located on the top right.
In addition to that, you will also notice that as
- acid strength decreases, conjugate base strength increases. That is the weaker the acid the stronger its conjugate base.
And as
- base strength decreases, conjugate acid strength increases. That is the weaker the base the stronger its conjugate acid.