Vapor pressure: what is it, how does it develop, and what can affect it?
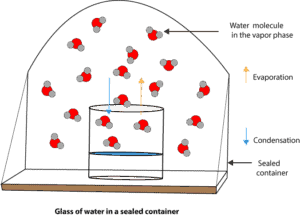
When the temperature of a liquid is held constant, its vapor pressure is the pressure exerted by its own vapor on its liquid phase when this vapor is in equilibrium with its own liquid phase. See the picture below:
How does this equilibrium form?
This equilibrium is formed when the rate at which molecules evaporate from a liquid is equal to the rate at which they condense back to liquid. For instance, we all know that when water is left in a glass cup on a countertop the water slowly disappears from sight and into the surroundings. But we also know that when we cover this glass cup with a container, the water molecules that break off from the rest will turn into gas and this gas will be trapped between the container and the space surrounding the cup and liquid from which they broke off.
As these trapped gas molecules constantly move and collide with each other and surface of liquid and walls of the container, some will transfer energy to others. As they continue to do this, some molecules will lose energy and become less energetic, while others will absorb energy and become more energetic. The slower and less energetic ones will become attracted by or stick to other water molecules in the liquid phase, turning them into liquid water as a result. This process of water vapor turning into liquid water is usually called condensation.
In the same way, some molecules in the liquid phase will absorb enough energy and move faster. As their motion or vibration grows, there comes a point where these water molecules break off their attractive forces between them and move away from each other. As they move away, the distance between water molecules increase and so these water molecules transition into gas. This process of water turning into water vapor is usually called evaporation.
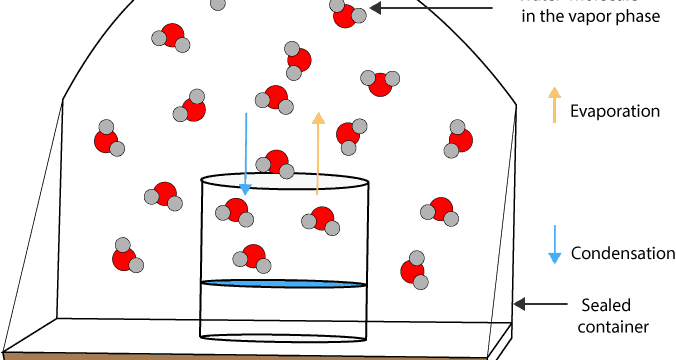
As molecules in the gas phase condense back into the liquid phase and molecules in the liquid phase evaporate back into the gas phase, there comes a time when the rate of condensation becomes equal to the rate of evaporation. At this point, chemists would say that the liquid and its vapor are in a state of dynamic equilibrium. Here is a picture to help us picture in our mind the molecular processes occurring at the molecular level.

The orange arrow shows water molecules evaporating, while the blue arrow shows water molecules condensing. Therefore, at dynamic equilibrium, the rate of water molecules condensing from the vapor phase into liquid is equal to the rate of water molecules evaporating from the liquid phase into gas. That is there is no net change in the relative amounts of water molecules turning into vapor and vapor turning back into liquid water. Thus, the molecular processes of evaporation and condensation of the liquid and its vapor are now in dynamic equilibrium.
What factors can affect the rate at which liquids evaporate?
The factors that can affect the rate at which liquids evaporate include:
- temperature
- liquid surface area
- strength of attractive forces between molecules (intermolecular forces)
How does temperature affect the rate at which water evaporates?
As temperature increase, the kinetic energy of water molecules also increases. This increase in kinetic energy enables water molecules break the attractive forces that hold them together in the liquid state. As more and more water molecules break these attractive forces, the more they gain the freedom to move and spread, transitioning into steam (gas).
How does surface area affect the rate at which water evaporates?
When the surface area of a liquid is wide, the more molecules will be exposed on the surface ready to evaporate. But these molecules will evaporate (transition to gas), if and only if they have sufficient energy to overcome the attractive forces between them. A way to think about surface area in this sense is to picture water in an open swimming pool and water in an opened tiny bottle.
How does strength of attractive forces affect the rate at which water evaporates?
When the attractive forces between water molecules is stronger, the rate at which water turns into steam (evaporates) is slower. This is because water molecules must absorb enough energy to increase their kinetic energy so that they can move away from each other. This moving away from each other is what we call breaking the attractive forces between them. But since this energy is hard to come by, only fewer water molecules will be successful at increasing their kinetic to such a point that it is sufficient to overcome these attractive forces.
Conversely, when the attractive forces between water molecules is weaker, the rate at which water turns into steam is faster. This is because many water molecules will be successful in absorbing energy sufficient to overcome these attractive forces.
A practical way to think about the role attractive forces play in evaporation is to picture rubbing alcohol sitting in an opened cup and water sitting in another. After one day, we will notice that the amount of rubbing alcohol left in the cup is smaller than the amount of water left in the other cup. This happens because the attractive forces between molecules in rubbing alcohol is weaker than the attractive forces between water molecules. These weaker attractive forces in rubbing alcohol causes it to evaporate faster than water.
To learn about why salt water has a lower vapor pressure than pure water, click here.