A stock or standard solution is a solution in which you accurately know its concentration. You can make stock solutions in the chemistry laboratory or buy from chemical manufacturers. Once you have a stock solution, you can prepare solutions of lower concentration by diluting the concentrated stock solution.
To dilute means to add a certain amount of solvent(water) to a certain amount of concentrated stock solution. If you add a certain amount of solvent to a certain amount of concentrated stock solution, you will notice that the amount of solute present in the stock solution is the same amount present in the dilute solution. The only difference is that the dilute solution now contains more water than the stock from which it was prepared. In real life, what you just read is similar to you adding more water to your coffee or tea to lighten its taste. As you add more water, you are only increasing the amount of water in the solution, but not the amount of coffee or tea molecules present in it. Here is an illustration of a dilute solution prepared from a stock solution:
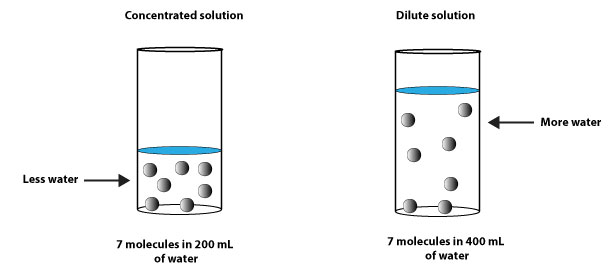
From the illustration above, you can see that there are 7 molecules of solute in the concentrated solution and 7 molecules in the dilute solution. But the dilute solution has more solvent in it than the concentrated solution.
Because both solutions contain an equal number of solute molecules, it follows that:
- the moles of chemicals present in the concentrated solution is equal to the moles of chemicals present in the dilute solution
If we translate the previous statement into a mathematical expression, we will get:
moles of concentrated solution = moles of dilute solution
But since we know that the amount of the coffee solution in moles is equal to the volume of the solution multiplied by its concentration in Molarity, then we can write that:
Volume (V) of concentrated solution times Molarity (M) of concentrated solution is equal to the volume (V) of dilute solution times the Molarity (M) of dilute solution. If we translate this into a mathematical expression, we will get:
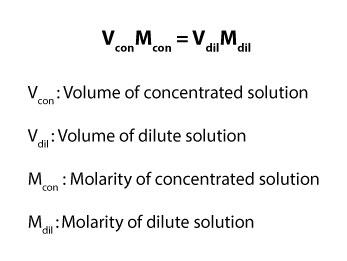
As you may have noticed, the formula we just derived is the general formula for diluting a concentrated solution to a solution of lower concentration. Note that if you report the concentration of solution as 2 M, the uppercase M is often used to represent the unit (mol/L), which is the unit for concentration reported in Molarity.
To use the dilution formula, you must know at least three of the four variables in it. Now, let’s used it to answer the following question:
Suppose that in one of your general chemistry experiments, you are required to use 2 M sulfuric acid (H2SO4) for a certain reaction, but your lab instructor gave you 5 M H2SO4. How would you go about this?
Solution
Since from the question you know the concentration of the sulfuric acid you need, then it follows that you must decide on what volume you need. So, let’s say you need 10 mL of 2 M H2SO4. Then it follows that you must calculate the volume of the stock solution you need to pipette in order to prepare your target concentration. To do this, you must recall the dilution formula. This formula says that:
- VolconMcon= VdilMdil
Now, if you read the question once again, you will notice that all the units are consistent with what we need to calculate. For this reason, there is no need to convert between units. Therefore:
Volcon=? (We need to calculate how much of the stock we need)
Voldil = 10 mL (This is the volume of the 2 M concentration you need)
Mcon= 5 M (This is the Molarity of concentrated solution)
Mdil= 2 M (This is the Molarity of the dilute solution)
If we substitute the above information into the dilution formula, we will get
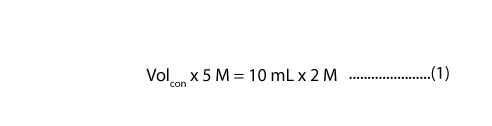
Since we need the volume of concentrated stock solution (Volcon), we must divide both the left and right side of the equal sign in the above equation (1) by 5 M. If we do, we will get:
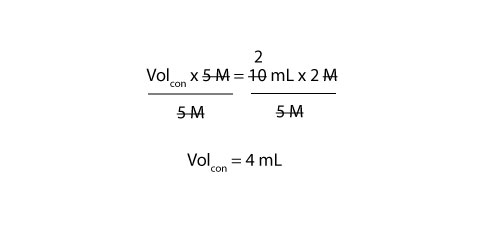
From the calculation, you need to pipette 4 mL of the 5 M sulfuric acid solution to prepare 10 mL of 2 M sulfuric acid solution. To prepare the 10 mL of 2 M solution, you must first transfer about 5 mL of distilled water into your 10 mL volumetric flask. Next, slowly add your 4 mL of stock solution (sulfuric acid). Swirl the flask and then top it up with more distilled water to the 10 mL mark.
Caution!
As you may have noticed, we added the sulfuric acid to the distilled water in the volumetric flask not the other way around. Why is that?
We did this in order to prevent the solution from exploding. As you may know, sulfuric acid is much denser than water, so as you add it to water, its molecules are able to travel within and mix well with the water molecules. However, because water is less dense than sulfuric acid, if you add it to sulfuric acid, its molecules will create a barrier in which you have water on top and sulfuric acid below. This unwanted barrier can cause the two chemicals to react explosively (exothermic reaction), generating enough energy to break the flask. In another way, you can prevent this reaction from happening when you place your volumetric flask in an ice bath while you add the acid to the distilled water. The ice will absorb the energy generated from the solution, as a result, cooling the solution down.
To learn how to prepare a solution from a solid substance, click here.