Percent means one part in a 100 or part in a whole. Therefore, percent composition of a compound is the percent by mass of each element in the total mass of a compound. Another way of saying the same thing is how much in percent each element contributes to the total mass of a compound. The higher the percentage composition, the higher the mass of the element present in the compound. Mathematically, we can express percentage composition as:

Now, let’s use the above formula to calculate the percentage composition of each element in water— H2O. From the chemical formula of water, we know that 1 mole of water contains 2 mol of hydrogen, H and 1 mol of oxygen, O. That is, we are assuming that:
1 mol of H2O contains 2 mol of H and 1 mol of O. But we do know that the molar mass of hydrogen is about 1.01 g/mol and the molar mass of oxygen is about 16.00 g/mol. Therefore, we can calculate the mass of 1 mol of water, H2O as:
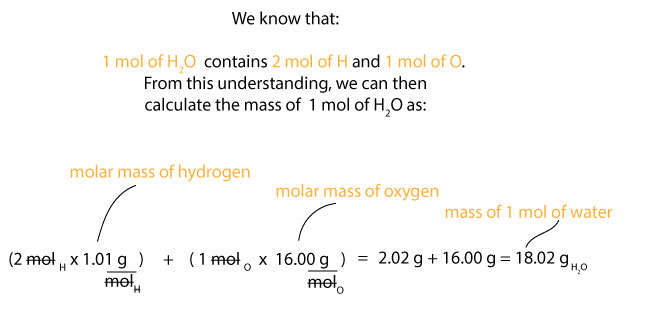
Now that we know the molar mass of water (whole), we can then calculate the percentage composition of hydrogen and oxygen in it as follows. For hydrogen, we know that its molar mass is 1.01 g/mol, but since we have 2 mol of it in water, then it follows that we must multiply the 2 mol of H by its molar mass. If we do, we will get 2.01 g, which is as a result of doing this: 2 mol x 1.01 g/mol = 2.02 g. We can then use this value, the molar mass of water, and the percentage composition formula depicted in equation 1 above to calculate the percentage composition of hydrogen in water. If you are following along, you will notice that we can set this calculation as:

Similarly, for oxygen, we know that its molar mass is 16.00 g/mol, but since we have 1 mol of it in water, then it follows that we must multiply the 1 mol of O by its molar mass. If we do, we will get 16.00 g, which is as a result of doing: 1 mol x 16.00 g/mol = 16.00 g. We can then use this value, the molar mass of water, and the percentage composition formula depicted in equation 1 above to calculate the percentage composition of oxygen in water. If you are following along, you will notice that we can set this calculation as:

From the calculation, you can see that the percentage composition of hydrogen and oxygen in water are 11.21% and 88.79% respectively. If you add these values together, you will notice they sum up to 100%. This means regardless of whether water is from mars or from earth, once it is pure water, its composition is fixed and will always consist of 11.21% H and 88.79% O.
Notice!
Since we know that you will get 100% when you add the percentage composition of all the elements in a chemical formula, then it follows that we can subtract the percentage composition of H from 100% to get that of oxygen. If you do, you will get:
- 100% -11.21% = 88.79%.
Once you know the percentage composition of all the elements in a chemical formula, except one. You can always use this shortcut to find its percentage composition.