Metallic bonding is an attraction between positively charged metal ions and a “sea” of surrounding negatively charged electrons. These valence electrons hold the positive ions together throughout the structure of the metal. So how does this arrangement allow metals like copper or aluminum wires conduct and carry electricity to our homes?
Metals are able to conduct electricity because atoms of metals easily lose their valence electrons to become positively charged ions. These positive ions of the metal are then surrounded by a “sea” of their valence electrons. When an electric field is applied to a metal, these electrons are able to move freely from atom to atom all the way to the positive end of the field, thus, conducting electricity as a result. This view of electrons moving freely from atom to atom is usually referred as the “electron sea” model. Let’s use the following picture to further illustrate this:
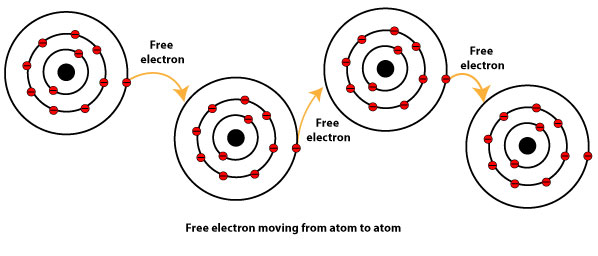
As you can see, an electron is freely moving from one atom to another. As one metal atom loses an electron and becomes a positively charge ion, it immediately accepts one from another to become a neutral atom. This back and forth losing and gaining of electrons among atoms of the metal allow metals to easily conduct electricity. However, some metals are better conductors of electricity than others.
Why is that?
This depends on how tightly metals hold their valence electrons. Metals that hold their valence electrons loosely are usually good conductors of electricity, while those that tightly hold their valence electrons are poor conductors of electricity. Best conductors of electricity include silver, copper, gold, and aluminum. And poor conductors of electricity include lead, tin and steel. Today, because of cost and weight, aluminum and copper are widely used conductors throughout the world. Aluminum is widely used by the electric power companies, while copper is widely used by the electronic industry.
As a result of the non-directional nature of metallic bond, metals generally exhibit the following properties:
- drawn out into thin wire (ductile) without losing its form
- hammered or pressed out of shape without breaking or cracking (malleable)
- conduct heat
- conduct electricity
On the other hand, what are insulators?
Insulators are materials that oppose the free movement of electrons. In other words, insulators hold their valence electrons tightly as a result they can’t move, or they require a large force to move them from atom to atom. Insulators in our everyday lives include rubber, glass, air, wood, oil, and ceramic.
If you like cooking, you will notice that cooking pots makers always take advantage of insulators and conductors to make our pots. For instance, the inside and outside of most cooking pots are often made of aluminum (good conductor of heat) so that energy can easily be transferred from the stove to cook our food, while the pot and lid handles are usually made of rubber or plastic (poor conductors of heat), so that we can easily lift or open out pots without getting burned.