A limiting reactant is a chemical in short supply during a chemical reaction. Usually, we can run a chemical reaction in one of two ways. The first is:
- using the exact mole amounts (stoichiometric) prescribed by the balanced chemical equation
while the second is:
- using a limited amount of one of the reactants while keeping the balanced chemical equation in mind. The reactant in limited amount is called the limiting reactant, while the reactant in excess is called the excess reactant.
What then is running a chemical reaction in a balanced way?
When we run a chemical reaction in a balanced way, we usually take the exact mole amounts of the reactants prescribed by the balanced chemical equation. For example, imagine that you are to run the following reaction in your lab:
H2+ I2 ——–> 2HI
which is the same as saying:
1 mol H2+ 1 mol I2 ——> 2mol HI
Since from the balanced chemical equation, the H2 and I2 are in a ratio of 1 mole-to-1 mole, it means that if we weigh exactly 1 mol of H2(2.02 g) and combine it with exactly 1 mol of I2(253.8 g), we will be running the reaction in a balanced way. When running a reaction in a balanced way, we have no clear limiting or excess reactant. Here is a picture do illustrate this point:
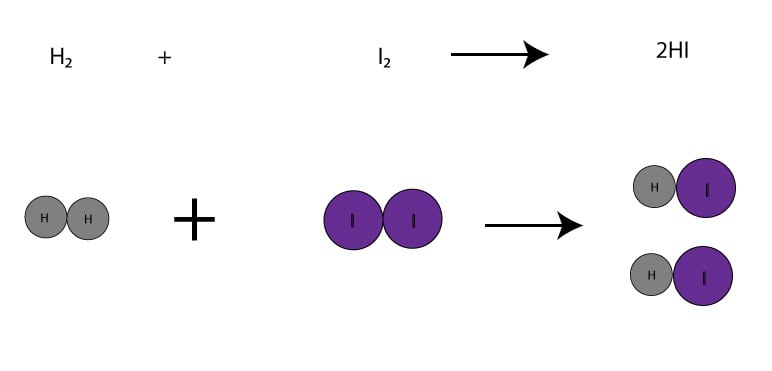
On the other hand, if we weigh 6 mol H2(12.12 g) plus 5 mol I2(1269 g), we will be running the reaction unbalanced. And since the moles of I2 is smaller than the moles of H2, the I2 will be the limiting reactant, while H2 will be the excess reactant. Let’s make a table to illustrate this:
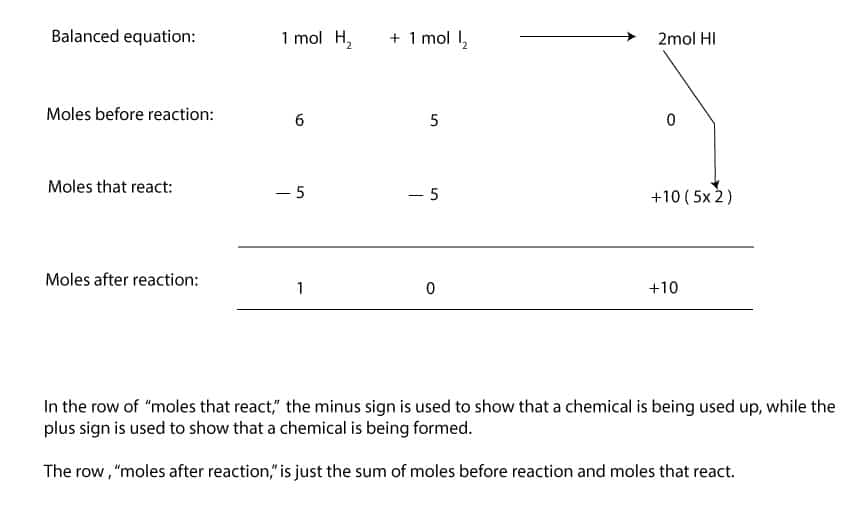
As you can see, H2 is the excess reactant, while I2 is the limiting reactant. But since 2 mol HI are produced from 1 mol H2 and 1 mol I2, it follows that we must multiply 2 mol by the moles that reacted, 5 moles, to get 10 moles of HI.
Here is a picture to illustrate the concept of limiting reactant:
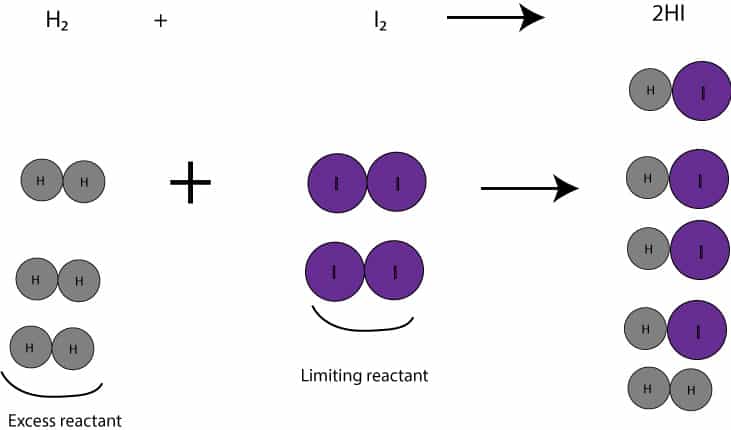
How to determine limiting reactant from a chemical equation?
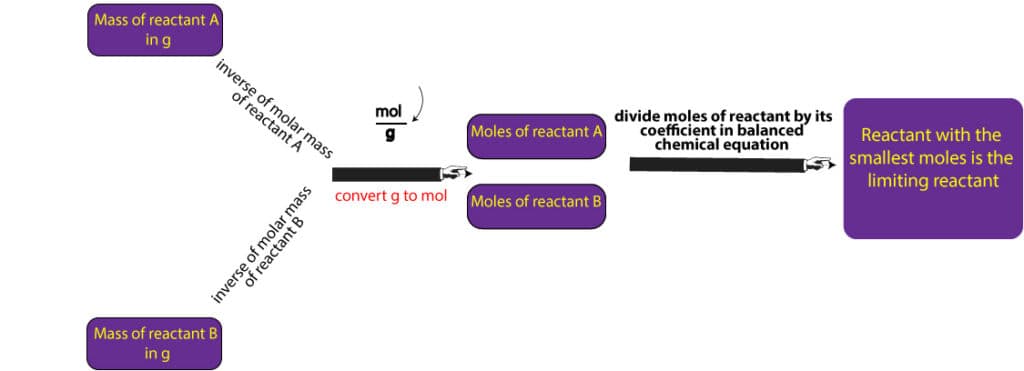
To determine the limiting reactant for a particular reaction, we must first ensure the equation describing the reaction is balanced. Once it’s balanced, we will then proceed to convert the grams of reactants to mole-coefficient ratios. Then, the reactant with the smallest ratio is our limiting reactant. In the previous example, we were given the amounts of reactants directly in moles, which is unusual. In chemistry, we are mostly given the amounts of reactants in grams, from which we are expected to convert to moles before we can determine the limiting reactant. Here are the steps to follow to do just that:
- calculate the molar mass of each reactant
- convert from grams of each reactant to moles of each reactant using the molar mass
- determine the mole-to-coefficient ratio by dividing the moles of each reactant by its corresponding coefficient from the balanced chemical equation
- reactant with the smallest mole-to-coefficient ratio is the limiting reactant
The above steps are also summarized in the following diagram:
Now, let’s apply the previous steps to solve the following problem
Question 1
Methane reacts with oxygen to produce carbon dioxide and water according to the following balanced chemical equation:
CH4+ 2O2 ———> CO2+ 2H2O
If 8.0 g of CH4 reacts with 64 g of O2, which of the reactants is the limiting reactant?
Strategy
Calculating the molar mass of each reactants (Step 1)
Since chemical equations speak in the language of moles, we must convert grams of each reactant to moles of each reactant (from step 1 above). To do this, we will first calculate the molar mass of CH4 and O2.
For molar mass of CH4, we will do: (1 x 12.01 g/mol) + (4 x 1.01 g/mol) = 16.05 g/mol
For molar mass of O2,we will do (2 x 16.00 g/mol) = 32.00 g/mol
Converting from grams of each reactant to moles of each reactant (step 2)
For moles of CH4, we will do:
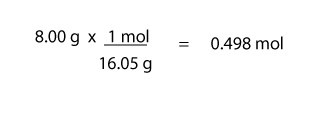
For moles of O2, we will do:
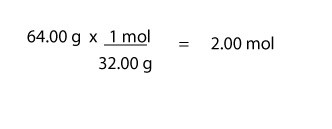
Determining the mole-to-coefficient ratio (step 3)
To determine the mole-to-coefficient ratio, we must divide the moles of each reactant from step 2 by its corresponding coefficient from the balanced chemical equation.
For CH4, its mole-to-coefficient ratio will be:
0.498/1 = 0.498
For O2, its mole-to-coefficient ratio will be:
2.00/2 = 1.00
Since the mole-to-coefficient ratio for CH4,is smaller than that of O2, then it follows that CH4 is the limiting reactant.
To calculate theoretical and percentage yield, click here
