To calculate percentage abundance, you must recall the atomic mass of an element is calculated by using the formula:

In the above formula you see fractional abundance. How do you get that? To get fractional abundance, you usually divide the percentage abundance of each isotope by 100. And when you add all the fractional abundance values of all the isotopes, you will notice they all add up to 1. To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100. Now, let’s apply our understanding to solve the following question:
Silver (Ag) has two stable isotopes: silver-107(107Ag) and silver-109 (109Ag). Silver-107 has a mass of 106.90509 amu and silver-109 has a mass of 108.90476 amu. Calculate the percentage abundance of each isotope.
Strategy
To calculate percentage abundance, we must first know the fractional abundance of each isotope. But from the question, we are not given these values, which means we must think of a way of finding them. One way we can find them is to remember that the:
fractional abundance of isotope 1 plus the fractional abundance of isotope 2 = 1
We know this because the sum of the percentage abundance values always equal 100. And since the fractional abundance is obtained by dividing the percentage abundance by 100, then, it follows that the sum of the fractional abundance must equal 1. Once we understand this, we can then let X represent the fractional abundance for isotope 1 and (1-X) represent the fractional abundance for isotope 2. Once we substitute these variables into the formula above, we will generate an algebraic equation from which we can solve to find X and then (1-X). Once we get the values for fractional abundance (X and 1-X), we can then multiply each fractional abundance by 100 to get percentage abundance.
Also, notice the question gave only the isotopic masses and not the atomic mass of silver. How do we get the atomic mass of silver to plug into the above equation? We get the atomic mass of silver by reading its value from the periodic table. And If you do, you will notice the atomic mass of silver is 107.8682. After gathering all the information necessary to solve the question, here is how the setup will appear based on the above information and formula:
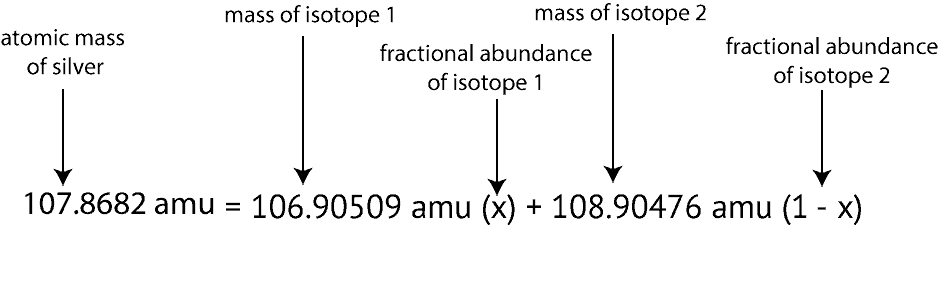
Next, we then apply our algebra skills to solve for X. To do this, we will expand the right side of the equation by multiplying X and 1-X by the numbers in front of them. If we do, here is what we will get:
107.8682 amu = 106.90509X amu + 108.90476 amu – 108.90476X amu
Next, we bring like terms together by moving 108.90476 amu from the right over to the left side of the equation. Since it’s a positive number on the right, it will become a negative number when it moves across the equal sign to the left. When we move it, here is how the rearranged equation will appear:
107.8682 amu – 108.90476 amu = 106.90509 X amu – 108.90476 X amu
Next, we subtract like terms
-1.03656 amu = -1.99967 X amu
Next, we divide by -1.99967 amu to isolate X.
-1.03656 amu/-1.99967 amu = -1.99967 amu X/-1.99967 amu
X = 0.518
Here is a more clear representation of the division step
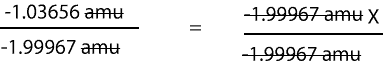
Solving for X, fractional abundance
X = 0.518
Notice that the negative signs and units cancel each other when we divide by -1.99967 amu, hence, fractional abundance has no units
X= 0.518 and 1 – X = 1-.518 = 0.482
Therefore, the fractional abundance of isotope 1 (Silver-107) is 0.518 and isotope 2 (Silver-109) is 0.482.
How to find percentage abundance
To get the percentage abundance, we will simply multiply each fractional abundance by 100. Recall that fractional abundance is calculated by dividing the percentage abundance by 100. Therefore, to get back percentage abundance, we multiply fractional abundance by 100. If we do, the percentage abundance for silver-107 is 0.518 x 100 = 51.8%. And percentage abundance for silver-109 is 0.482 x 100 = 48.2%
To learn how to calculate atomic mass using percentage abundance and isotopic masses click here.