The ionization energy (IE) of an atom is the energy required to remove an electron from an atom in a gas phase. How an atom reacts with another partly depends on how easy it is to remove its valence electrons. Because energy is always put in (endothermic process) to remove an electron, values of ionization energies are usually positive. For this reason, the more tightly the nucleus of an atom attracts its valence electrons, the more difficult it is to remove them. And the more difficult it is to remove them, the higher the ionization energy.
For elements in the s and p block of the periodic table, the ionization energies required to remove an electron from the neutral atom (called first ionization) usually decreases down a group but increases across a period. Here is a plot of the first ionization energies of elements in the first and second period, however:
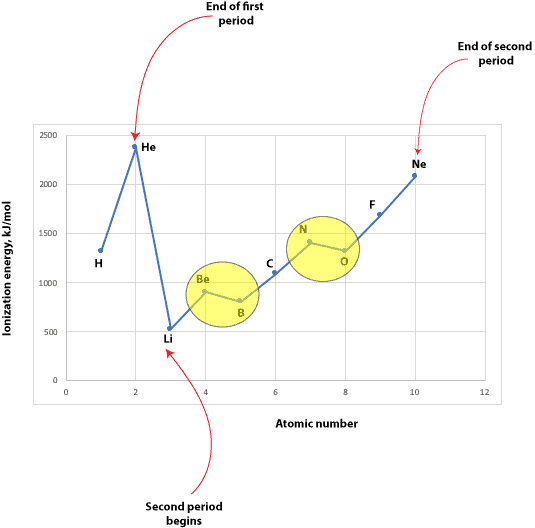
When you look at the graph, you will notice that the first ionization energies don’t increase smoothly along the second period. That is, there are interruptions at the elements in the yellow circle. So, the question is why is that? To answer that, we must consider the electron configurations of the elements at these interruptions.
First interruption,
Why is the first ionization energy of Boron (B) lower than that of Beryllium (Be)?
To answer that, let’s compare their electron configurations:
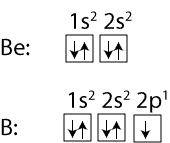
As you can see, in Be, the 1s and 2s orbitals are completely filled with electrons, while in B, the 2p orbital has only one electron in it. For the first ionization of Be, a 2s electron is removed, while for the first ionization of B a 2p electron is removed. Since the 2s electron is closer to the nucleus, its electron is much strongly attracted to the nucleus than the electron in the 2p orbital. Because of this, it’s much easier to remove an electron from B than it is to remove from Be. Therefore, Beryllium has a higher first ionization energy than B.
Second interruption
Why is the first ionization energy of Oxygen (O) lower than that of Nitrogen (N)?
To answer that, let’s compare their electron configurations:
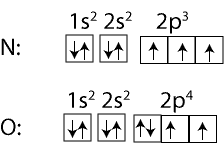
As you can see, if we follow Hund’s rule of filling orbitals of equal energy (degenerate orbitals) with electrons, you will notice that the p orbitals in Nitrogen will have one electron each and these electrons will have the same spin (pointing in the same direction) and repel each other to some extent. However, in oxygen the repulsion is greater. This is, because one of the p orbitals has two electrons in it. These electrons repel each other in addition to the repulsion from the other electrons. Because of this, it’s much easier to remove an electron from oxygen than it is to remove from nitrogen. Hence, nitrogen has a higher first ionization energy than oxygen even though in both atoms a 2p electron is removed.
Why does first ionization energy decrease down a group?
It decreases because as the atoms get larger the inner electrons tend to repel the valence electrons strongly weakening the nucleus attraction for the valence electrons. Because of this, it’s much easier to remove an electron.
If you want to learn more about electron affinity and how it trends on the periodic table, click here.