Formal charge is the charge we assign to a bonded atom if the bonding electrons were shared equally between the bonded atoms.
Why do we have to assign formal charges?
Sometimes we can write more than one Lewis structure for a particular ion or molecule. When that happens, we usually assign formal charges to the bonded atoms to help determine the correct Lewis structure.
To determine the formal charge for an atom, we usually follow these rules:
- Assign all lone pairs of electrons to the atom on which we find them
- Assign half of the bonding electrons to each atom in the bond
After applying the rules outlined above to each atom in the Lewis structure, we will then use the following formula to calculate the formal charge of each atom:

Once we add all the formal charges for the atoms in the Lewis structure, we should get a value equal to the actual charge of the molecule or ion. If it is a neutral molecule, then the sum of all the formal charges must equal zero. If it is a molecular ion, then the sum of all the formal charges must equal the ionic charge.
How to decide the correct Lewis structure after assigning formal charges
After assigning formal charges, we again apply the following rules to identify the correct Lewis structure:
- Negative formal charge should be on the most electronegative atom
- Like charges should not be on adjacent atoms
Now, let’s apply the above rules to predict the best Lewis structure for the molecule,
Hydrogen Cyanide (HCN).
To draw the Lewis structure for HCN, we will first calculate the total number of valence electrons. If you look on the periodic table, you will notice that H has one valence electron, C has 4, and N has 5. If you sum all these valence electrons, you will get 10. Next, we identify the central atom. But we know that hydrogen can form only a single bond, this means H cannot be the central atom. Since H cannot be the central atom, it follows that only C or N can be the central atom. If carbon is the central atom, then the bond skeleton of its Lewis structure will appear as:
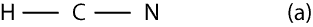
If N is the central atom, then the bond skeleton of its Lewis structure will appear as:

Since the bond skeleton consists of 4 electrons, it follows that we have 6 electrons (10 – 4) leftover to distribute. To distribute the 6 electrons, we start from the outer atoms, but since H can have only 2 valence electrons and it does have its 2 valence electrons, we move on to N in the bond skeleton (a). As you can see, N needs only 6 more electrons to have an octet, so we give all the 6 electrons to it. Therefore, the Lewis structure of (a) will appear as:
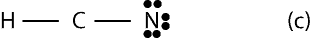
However, notice Carbon has only 4 electrons, but needs 4 more to satisfy the octet rule. This means, we must move two lone pairs of electrons from N to form multiple bonds between C and N. If we do, the completed Lewis structure with carbon in center will appear as:

And the completed Lewis structure with N as the central atom (b) will appear as:

So, the next question is which of the Lewis structures is the correct one?
To determine that, we must assign formal charges. So, let’s start with Lewis structure (e). To determine the formal charge of H, we must first figure out how many electrons it owns in the Lewis structure. From the rules outlined above, we do know it owns half of the shared electrons between it and C. And since there is a single bond between it and C, it follows that H owns only 1 electron (2 electrons divided by 2). Now, to determine the formal charge of H, we will simply subtract 1 from the valence electron of H predicted by the periodic table. If we do, we will get: 1-1 = 0. Therefore, the formal charge of H is zero.
Similarly, formal charge of C will be: 4 – 4 = 0. And formal charge of N will be: 5-5 = 0 (recall to count the lone pairs on N)
If we include the formal charges to Lewis structure (e), it will appear as:
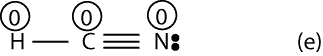
If we calculate the formal charge for Lewis structure (f), we will get:
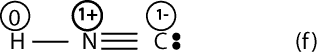
Now, if we look at Lewis structures (e) and (f) with formal charges, we can predict with reason that structure (e) should be stable. We think so because all the atoms in (f) have a formal charge of zero. However, in structure (f) notice that N has a formal charge of 1+, while C has a formal charge of 1-, but N is more electronegative than carbon. Since the negative charge should reside on the most electronegative atom, if follows that Lewis structure (f) is incorrect (unstable).