- What’s the difference between Rutherford and Thomson’s atomic model?
Atomic model based on:
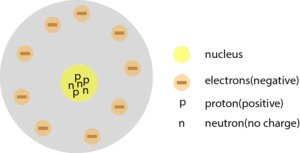
Rutherford : Has a nucleus with protons and neutrons. Protons carry a positive charge, while neutrons have no charge.
Here is Rutherford’s atomic model
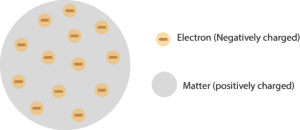
Thomson: Has no protons nor neutrons.
Here is Thomson’s atomic model