What’s the periodic table?
The periodic table is a systematic arrangement of elements according to their properties and atomic number.
Who formulated the periodic table?
A Russian scientist called Dimitri Mendeleev formulated the first periodic table.
How did Mendeleev formulate the periodic table?
Mendeleev observed that the atomic weight of an element is related to its physical and chemical properties.
How did he figure this out?
Mendeleev lined all the elements that existed at that time according to their atomic weight. He then compared their physical and chemical properties to one another. He noticed that a chemical behavior that was seen in one element was also seen in another. This repeated pattern of chemical behavior is called periodicity or periodic behavior. It is because of this periodic behavior that the table of elements is called the periodic table.
Here is how Mendeleev discovered periodicity
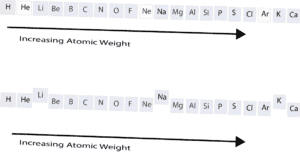
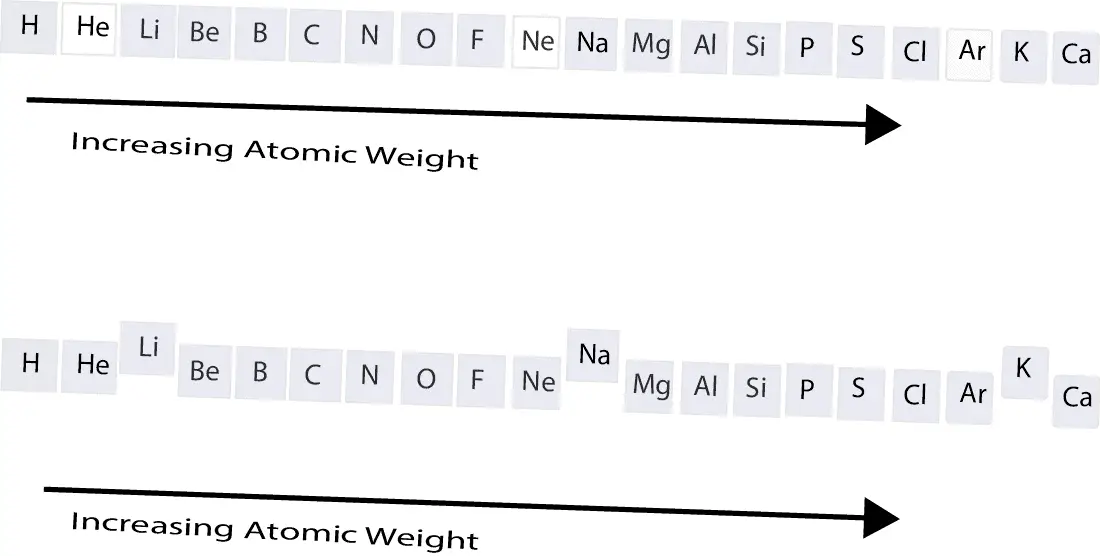
From the first model, you can see that the elements are lined up according to increasing atomic weight. You will also notice that the elements with the white background (He, Ne, Ar) are noble gases. These elements are stable and unreactive under normal conditions. Because they share the same chemical properties, Mendeleev decided to put them in the same group or family. This same approach was used to put Li, Na, and K in the same family.
What’s the law of octaves?
Mendeleev observed that the properties of elements repeated 8 elements away to the left or right of an element when arranged by atomic weights. This pattern of 8 was called the “law of octaves”. For this reason, Mendeleev had eight columns on his periodic table. Later, other scientists discovered the pattern of 18 and 32. As transition elements started making their way into the periodic table starting on the fourth row (period 4), the chemical properties repeated after every 18 elements (8 main group elements plus 10 transition elements). Similarly, when the Lanthanoids and Actinoids are included in periods 6 and 7, the chemical properties of the elements repeated after every 32 elements. To learn more about Mendeleev’s periodic table, click here.
Why did scientists believe in Mendeleev’s periodic table?
Mendeleev left gaps for unknown elements yet to be discovered to fill those positions. And when these elements were later discovered, their properties were almost identical to what Mendeleev had predicted.
How different is the modern periodic table to that of Mendeleev’s?
Although we now have more elements (118) than Mendeleev’s time, his way of organizing the elements is still the same today. However, on the modern periodic table the elements are now ordered using increasing atomic number. Since atoms of the same element can have different atomic weights (isotopes), using atomic number is a more accurate measure than using atomic weights. As you recall, atomic number measures the amount of positive charge in the nucleus of an atom. And the atomic number gradually increase as you move from one element to the next. Now let’s use the periodic table below to identify its parts.
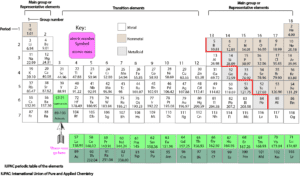
Here is another periodic table to show how the elements are organized into blocks:
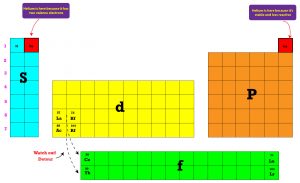
How’re the elements grouped in the periodic table?
As you can tell, the elements are arranged in vertical columns called groups or families. And in horizontal rows called periods. The groups are numbered from 1 through 18, and the periods from 1 through 7. Elements in the same group or family usually share similar chemical properties and some physical properties. These elements are further classified into main group (representative) and transition elements. The main group elements show the strongest periodic relationships than the transition elements. As you look on the table, you will notice that the background of the elements are shaded differently. This shading is used to classify the elements into metals, nonmetals and metalloids.
Where are the metals, non metals and metalloids located on the periodic table?
Except hydrogen, generally, nonmetals are found on the right side of the periodic table. Hydrogen is put in group 1 because it behaves chemically like the other members of the group. You will also notice that the nonmetals are fewer than the metals.
Metals are located on the left side of the periodic table. Metalloids are located on the zig-zag line on the period table.
What’s the zig-zag line used for?
The zig-zag line (red) is used as a device to help locate the regions for metals, nonmetals and metalloids.
Why’re the two rows of elements hanging at the bottom of the periodic table?
The two rows hanging at the bottom belong to a special group of elements called Lanthanoids and Actinoids. These two groups are still metals and they fall under group 3. The reason they hang at the bottom of the table is to ensure that the periodic table can fit onto a single page.
What do the numbers above and below each chemical symbol represent?
The top number is the atomic number, and all the elements on the periodic table must have this number. The bottom number is the atomic mass (atomic weight) of the element. Scientists determine the atomic mass of an element using the most stable versions (isotopes) of the element. If scientists can’t find stable versions of the element, then they can’t accurately determine the mass of the element. As a result, some of the elements have their atomic masses missing on the IUPAC periodic table. To learn how scientists determine atomic mass, click here.
What’re the general properties of metals?
These properties include:
- Except mercury, all the metals are solids at room temperature
- Shiny
- conduct heat and electrical current
- Malleable (can be stretched or shaped into different forms)
- Ductile (can be drawn out or hammered thin)
- Lose electrons to form cations (positively charged ions)
What’re the general properties of nonmetals?
These properties include:
- Poor conductors of heat and electricity
- can exist as solids, liquid or gases
- Nonmetals that exist as solids are brittle
- Gain electrons to form anions (negatively charged ions)
What’re the general properties of metalloids?
These properties include:
- Poor conductors of heat and electricity
- Exist as solids at room temperature
- Exhibit the properties of metals and nonmetals