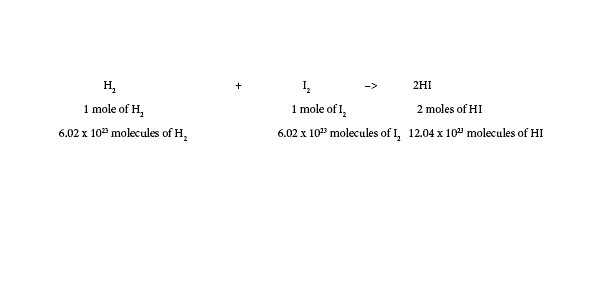
First, a molecule consists of at least two or more atoms bonded together.
Here is an example of a molecule:
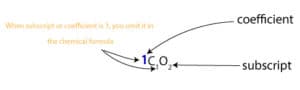
As you can see, a molecule usually consists of coefficients and subscripts. The coefficients can be interpreted as molecules or moles. While the subscripts can be interpreted as atoms or moles.
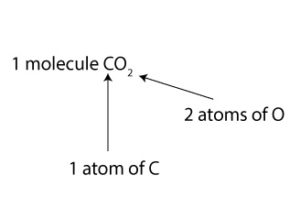
To interpret the chemical formula in terms of molecules, we will say 1 molecule of CO2 consists of 1 atom of carbon (C) and 2 atoms of oxygen (O). Here is a picture of the interpretation:
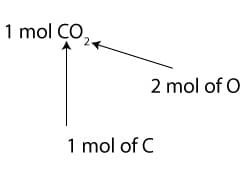
To interpret the chemical formula in terms of moles, we will say 1 mol of CO2 consists of 1 mol of carbon (C) and 2 mol of oxygen (O). Here is a picture of the interpretation:
To learn how to use a chemical equation to convert from moles of one chemical to moles of another, click here.