What is molar mass and how do you calculate it?
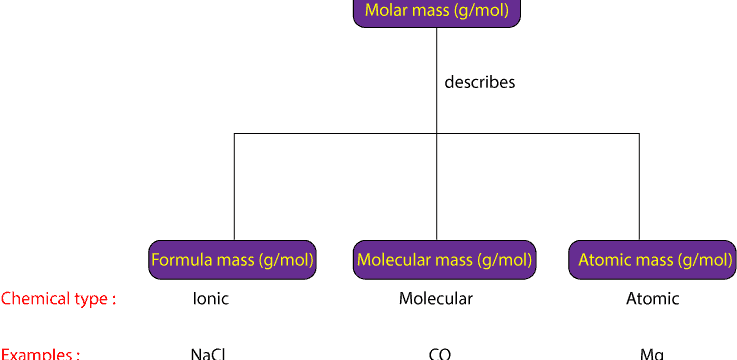
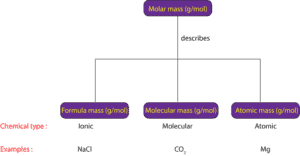
Molar mass is the general term used to describe the mass in grams of one mole (mol) of a chemical substance. Usually, molar mass has units of grams per mole (g/mol). However, chemicals can vary based on their internal structure. Some chemicals consist of molecules. Some chemicals consist of atoms. Some chemicals consist of formula units.
If a chemical substance consists of molecules, its mass in grams of one mole of its molecules is usually referred as its molecular mass.
If the chemical substance consists of atoms, its mass in grams of one mole of its atoms is usually referred as its atomic mass.
If the chemical substance consists of formula units, its mass in grams of one mole of its formula units is usually referred as formula mass.
What are formula units?
Formula units usually consist of positive and negative ions held together in a repeating three-dimensional pattern. This type of arrangement is common in ionic compounds such as sodium chloride and others. So, instead of us using atomic, molecular, or formula mass to describe the mass of a type of chemical substance, won’t it be simpler if we could use one term to describe the mass of one mole of atomic, molecular or ionic compounds? So, that is where the term molar mass comes in. Regardless of type of compound, you can used it to describe the mass of one mole of a chemical substance. Here is an illustration of how molar mass is related to atomic, molecular, and formula mass:
How do you calculate the molar mass of an element or compound ?
To calculate molar mass, you need to know at least two things. These are:
- name and chemical symbol of element or chemical formula of compound
- atomic mass of the elements from the periodic table
For names of elements and their atomic masses, we can easily find that information from the periodic table. For chemical formula of chemical substance, we are usually given that information in the question or asked to deduce it from given information. Now, let’s apply these principles to work the following examples:
Example
Calculate the molar mass of sodium (Na)
Solution
From the question, we have only one atom of Na. This means, we simply locate Na on the periodic table and read its atomic mass. If you read it correctly and rounded it to two decimal places, you should get 22.99. Now, to get its molar mass, we must attach the unit grams per mol to its atomic mass. Thus, molar mass of sodium (Na) is 22.99 g/mol. Grams per mol is another way of saying that for every 1 mole of Na, there are 22.99 g of sodium atoms in it. If you are confused about this, click on this link to review the mole concept.
How to calculate the mass of a hydrate
Example
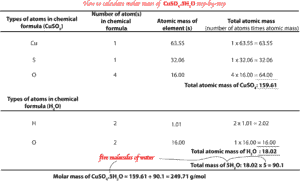
Calculate the molar mass of CuSO4.5H2O (copper (II) sulfate pentahydrate)
Solution
As you can see, this formula is a bit complex, but let’s break it down to manageable chunks. So, what does the chemical formula tell us? This chemical formula (CuSO4.5H2O) tells us that in the chemical, copper (II) sulfate (CuSO4), there are five molecules of water (5H2O) in its crystal structure. As you can see, the number 5 in front of water (5H2O), only affects water. This means after calculating the mass of water (H2O), we must multiply the mass by 5 to get the total mass of 5 molecules of water, and then add this value to the mass of CuSO4 to get the total mas of CuSO4.5H2O. Having said all that, let’s now construct a table to calculate the molar mass of CuSO4, and another to calculate the mass of 5H2O. After calculating the masses of both, we will then add them together to get the molar mass of CuSO4.5H2O. Remember that molar mass of CuSO4.5H2O is simply the sum of the atomic masses in the chemical formula with the units of grams per mol attached to it. Here is the table:
As you can see, the table is self-explanatory. The only thing to remember is that to get the total atomic mass for each type of element, you must multiply the number of atoms of that element in the chemical formula by its corresponding atomic mass from the periodic table. If a particular element has no subscripts written beside it, it usually means that only one atom of that element is present in a molecule or formula unit of that chemical.