Buffer solution: what is it and how does it work to resist changes in its pH?
A buffer is a solution that resists changes in its pH when small amounts of strong acid or base is added to it. Small amount is bolded to stress the fact that if you add too much strong acid or base to your buffer, it’s pH will change. This means buffer solutions have a limit to how much acid or base you can add to it without changing its pH that much. This limit is usually called the buffer capacity.
If this limit is too high because of the high concentration of buffer components, then its buffering capacity will be high as well. If this limit is too low because of the low concentration of buffer components, then its buffering capacity will be low as well. This means the buffer capacity is affected by the relative concentrations of the buffer components. As buffer works to resist changes in its pH, the concentration of one component decreases, while the concentration of the other increases.
What are the components of a buffer solution?
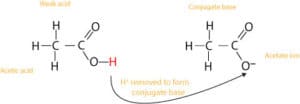
To understand how buffers work, we must first discuss the concept of conjugates in acid-base chemistry? What is a conjugate? A conjugate means a “mate.” If we translate this meaning to acid-base chemistry, then we can say that every acid is tied to its mate called “conjugate base,” and together, they are called “conjugate acid-base pair.” Similarly, every base is tied to its mate called “conjugate acid,” and together, they are called “conjugate acid-base pair.” Here is a model showing this relationship between acetic acid, CH3COOH and its conjugate base, acetate ion, CH3COO–:

From this, you will notice that a conjugate pair always consists of an acid and a base. And when a proton(H+) is removed from an acid, the acid turns into its conjugate base. And when a proton is added to a base, the base turns into its conjugate acid. Thus, the acid and its conjugate base are related by the loss and gain of a proton.
If we apply the concept of conjugate to buffer solutions, then we can say that a buffer solution usually consists of a weak acid and its conjugate base or a weak base and its conjugate acid. However, not all acids and their conjugate bases can form buffer solutions. For instance, on paper, the conjugate base of hydrochloric acid (HCl) is the chloride ion (Cl). Yet, in real life, the chloride ion is usually reluctant to accept a proton and turn into its conjugate acid, HCl. Because of this behavior, strong acids and their conjugate bases do not usually form buffer solutions. So too do strong bases. As a result, buffer solutions usually consist of a mixture of weak acids and their conjugate bases and weak bases and their conjugate acids.
Here is an example of a weak acid—acetic acid with its conjugate base—acetate ion. Together, they can form a buffer:
At the molecular level, how do buffer solutions work to resist changes in pH?
To understand how buffer solution resist changes in pH, we must first consider the dissociation of weak acid in water. That is, if we represent a weak acid by HA, then we can say that this weak acid (HA) dissociates in water to produce hydronium ions (H3O+) and its conjugate base, A–. However, since HA is a weak acid, only some of its molecules will dissociate. This means HA will not dissociate 100% in water (see double arrow). As a result, we can write the following equations to describe the dissociation of weak acid in water:
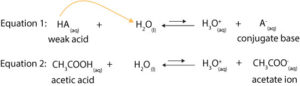
From equation 1, you will notice that the weak acid, HA donates a proton to water and then turns into its conjugate base, A–. Thus, the acid, HA is in equilibrium with its conjugate base, A–. Similarly, from equation 2, you will notice that the weak acid, acetic acid is in equilibrium with its conjugate base, acetate ion. Now,
How does buffer solution resists changes in its pH?
When you add small amounts of strong base (OH–) to a buffer, the buffer will resist changes in its pH by sending an equal amount of its weak acid to donate a proton to the base. Once the base accepts the proton, it turns into water, while the weak acid turns into its conjugate base. Here is an equation showing this behavior:
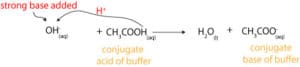
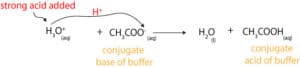
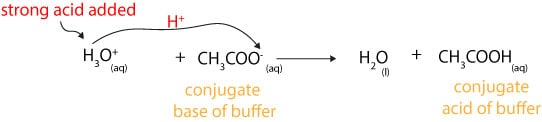
Similarly, when you add small amounts of strong acid (H3O+) to a buffer, the buffer will resist changes in its pH by sending an equal amount (stoichiometric amount) of its weak base to accept a proton from the strong acid. Once the base accepts the proton, it turns into its conjugate acid, while the strong acid turns into water. Here is an equation showing this behavior: