How do you prepare a buffer solution of known pH from scratch?
To prepare a buffer solution of known pH from scratch, we must carry out the following steps:
- choose a conjugate acid-base pair
- calculate the ratio of buffer components
- determine the buffer concentration
- mix and adjust the buffer’s pH
Step 1: choosing the conjugate acid-base pair
First, we must decide on the pH before choosing the conjugate acid-base pair. For example, imagine that we need a buffer solution of pH 6.3. Then it follows that we must choose an acid component with a pKa close to 6.3. Since pKa= -logKa, then it follows that Ka= 10-6.3= 5.01 x 10-7. Armed with this information, we will then scan through a table of acid-dissociation constants to find an acid with a Ka close to 5.01 x 10-7. In our example, we found that carbonic acid (H2CO3) and hydrogen carbonate ion (HCO3–) with a pKa of 6.38 are a suitable match. However, the hydrogen carbonate ion will be supplied by a salt such as sodium hydrogen carbonate (NaHCO3).
Step 2: calculating the ratio of buffer components
To calculate the ratio of buffer components, we must use the Henderson-Hasselbalch equation. In the equation, we will plug in the pH and pKa and then solve for the ratio. Here is the setup:
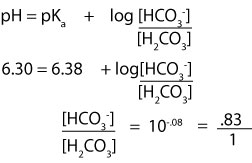
From the ratio, you can see that for every 1.0 mol of carbonic acid in a liter of solution, you will need 0.83 mol of NaHCO3.
Step 3: determining the buffer concentration
We will need to decide the concentration of the buffer solution. But recall that the higher the concentration of the buffer components the stronger the buffer capacity. For our example, imagine that we have in stock 0.5 M carbonic acid but needs 1L of HCO3–/H2CO3 buffer. To prepare this buffer, we will need to figure out in grams how much NaHCO3 we will need to add to the 0.5 M carbonic acid to achieve the 6.3 pH. To do this, we must first calculate the moles of H2CO3. Next, use the buffer ratio from the Henderson-Hasselbalch equation in step 2 to convert moles of H2CO3 to moles of NaHCO3, and finally, convert moles of NaHCO3 to grams of NaHCO3. Here is the setup of such a calculation:
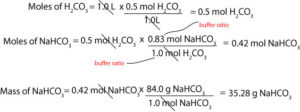
Notice that to get the mass of NaHCO3 we must multiply moles of NaHCO3 by its molar mass.
Step 4: Mixing the components to form the buffer solution
From step 3, we will weigh and dissolve the 35.28 g of NaHCO3 in enough 0.5 M H2CO3 and then add more 0.5 M H2CO3 to bring the solution to a total volume of 1.0 L. Next, we will check the pH with a pH meter to see whether we have the desired pH. If the buffer’s pH is slightly higher than what we expect, we will add a strong acid dropwise until we reach our desired pH. Similarly, If the buffer’s pH is slightly lower than what we expect, we will add a strong base dropwise until we reach our desired pH.
Since the NaHCO3 is an ionic compound, it completely dissolves and dissociates into sodium (Na+) and hydrogen carbonate ions. Once it completely dissolves, the sodium ions in the solution contribute little to the buffer capacity. For this reason, the sodium ions are usually called “spectator” ions. Spectator in the sense that the sodium ions watch while the carbonic acid and hydrogen carbonate ions consume the added strong acid or base in the buffer.