Energy can be transferred by heat in three ways. These are:
- conduction
- convection
- radiation
This transfer usually occurs because of a difference in temperature between the things (systems) involved.
How is energy transferred by conduction?
At the macroscopic level, a piece of iron sitting on a table may appear motionless to our eyes. However, at the microscopic level, this piece of iron consists of tiny invisible particles called atoms. These atoms are constantly vibrating inside this piece of iron. As they vibrate, these atoms and their electrons transfer energy to other neighboring atoms. Let’s use the following illustration to further explain this behavior:
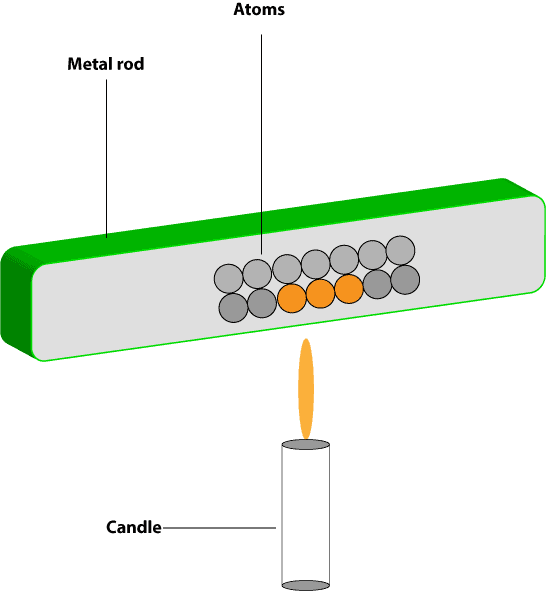
As you can see from the picture above, the iron rod consists of many iron atoms inside it. As the iron rod is heated in the candle’s flame, the iron atoms closer to the flame gets heated first, as a result, they vibrate more. As these atoms become warmer and vibrate more, they intend transfer energy to other nearby atoms in contact with them. This process of energy transfer continues until energy passes through the whole iron rod. Recall that the natural principle behind this energy transfer is that: energy can only be transferred from a higher temperature to a lower temperature. This implies that: the candle’s flame is at a higher temperature than the temperature of the iron rod. As a result of this temperature difference, energy is transferred from the candle’s flame to the atoms of the iron rod closest to it. As these atoms become warmer, they intend transfer energy to the atoms closest to them. This process of energy transfer continues throughout the whole rod, allowing energy to pass through the whole iron rod. If the rod becomes warmer than the air surrounding it, it will intend transfer energy to the air molecules surrounding it. And these air molecules will intend transfer energy to other air molecules. This process of transfer continues until the substance transferring the energy and the substance absorbing it reach the same temperature. Once they reach the same temperature, the energy transfer stops. Note that energy transfer that occur by the vibration of atoms and flow of electrons is called conduction. And for conduction to occur, the things (systems) involved must be near each other and in contact. This is why energy transfer by conduction is common in solids. Because in most solids, the atoms are tightly and closely packed.
How is energy transferred by convection?
At the macroscopic level, your drinking water sitting in your aluminum cup may appear motionless. However, at the microscopic level, this liquid water consists of tiny invisible water molecules jostling and sliding over one another. Now, what happens to the water molecules when you place a burning candle under the cup? Once you place a burning candle under it, energy will first be transferred from the burning candle to the water molecules closest to the flame. Let’s use the following illustration to further explain this behavior:
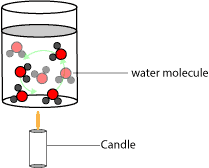
As you can see, energy is first transferred from the candle’s flame to the water molecules near it—water molecules at the bottom of the cup. Once these water molecules are warmed up, they move faster and their kinetic energy increase and so they move up (to the surface) and slower and less warmer water molecules move in to fill their old positions at the bottom of the cup. Once these water molecules are also warmed up, they also move to the surface and less warmer water molecules move in to replace them at the bottom of the cup. This cycle continues back and forth to allow energy pass through the whole liquid. Note that energy transfer that occur by the movement and flow of molecules is called convection. And for convection to occur, the things involved (systems) must move and should be near each other. This is why energy transfer by convection is common in liquids and gases. Because in liquids and gases, the molecules are mobile and reasonably close.
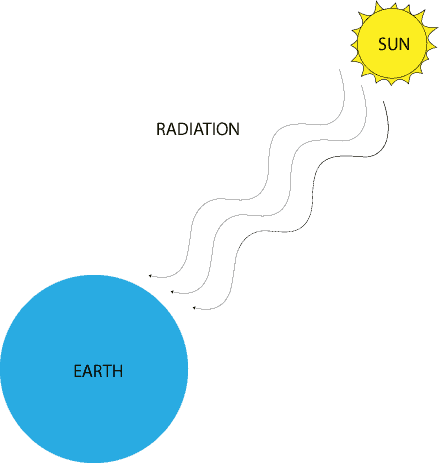
How is energy transferred by radiation?
At the macroscopic level, we all feel the intensity of the sun’s energy hitting us on a hot day. What most of us don’t realize is that this energy from the sun reaches us by travelling through mostly empty space (vacuum). In other words, radiation is a way in which energy travels in the form of waves through a vacuum. Unlike conduction and convection in which the systems involved in the energy transfer must be reasonably close. In radiation, closeness is not necessary, because electromagnetic waves can travel through a vacuum. Let’s use the following illustration to picture how energy travels by radiation: