Salt water has a lower vapor pressure than pure (distilled) water because salt water evaporates at a slower rate than pure water. Let’s use the following illustration to further explain this:
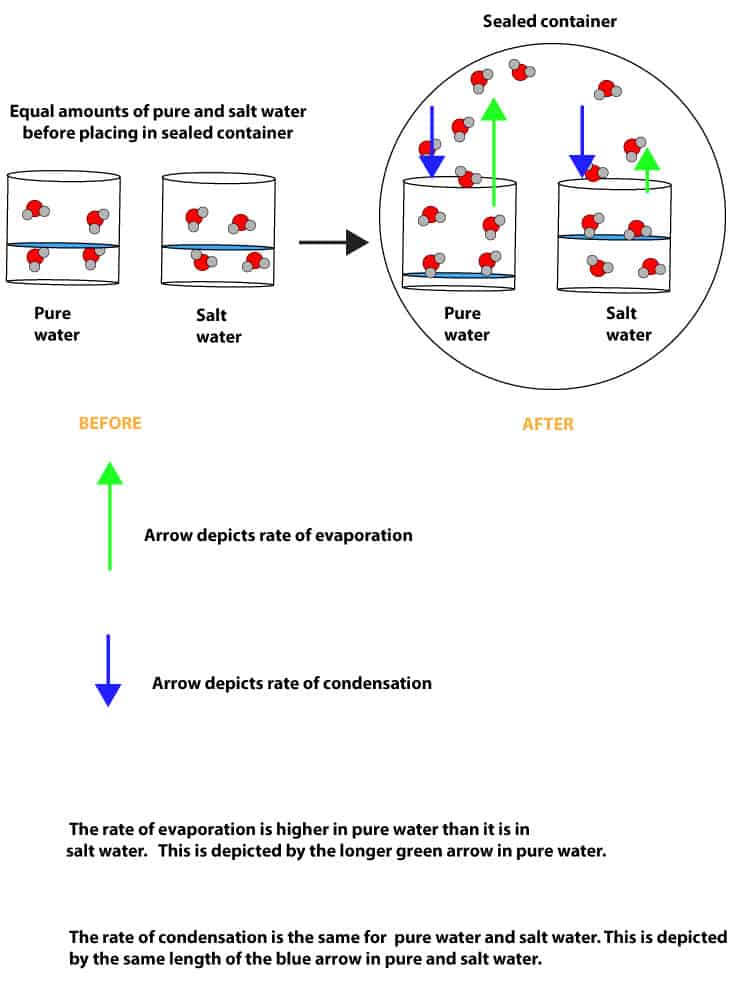
From the above illustration, imagine that you fetched 200 mL of pure water into one beaker and another 200 mL of salt water into another beaker. You then placed both beakers in a sealed container and then recorded your observations over time. As you recorded, you noticed something interesting: you noticed that the amount of pure water kept decreasing while the amount of salt water kept increasing. Why was that? This was so because of two main reasons. First, the water molecules in pure water were able to overcome the attractive forces existing between them quicker than the water molecules in salt water. As a result, they were able to evaporate faster than the water molecules in salt water. In other words, salt water evaporates slower than pure water. Because of this, the vapor pressure of pure water was higher than the vapor pressure of salt water.
Second, while the rate of evaporation was higher in pure water, the rate of condensation was the same for both pure water and salt water. This was so because water molecules in the vapor phase had an equal chance of condensing back into either the pure or salt water in the sealed container. This means that as water evaporates and condenses from both containers overtime, there will always be more salt water than pure water in their respective beakers. However, at a given temperature, the vapor pressure of salt water will always be lower than the vapor pressure of pure water.
In sum, the vapor pressure of an equal amount of pure water will always be greater than the vapor pressure of an equal amount of salt water.
To learn more about vapor pressure, click here.