The vapor pressure of solution depends on the concentration and NOT the identities of the solute particles in the solution. This is because adding a non-volatile solute such as salt to pure water changes the physical properties of pure water. And the more salt you add, the more you will change its physical properties. The relationship between vapor pressure of solutions and the concentrations of its non-volatile solutes was explored by a scientist called Raoult.
Raoult discovered that the vapor pressure of solution is equal to the mole fraction of the solvent multiplied by the vapor pressure of the pure solvent. The resulting relationship called Raoult’s law can be expressed mathematically as:
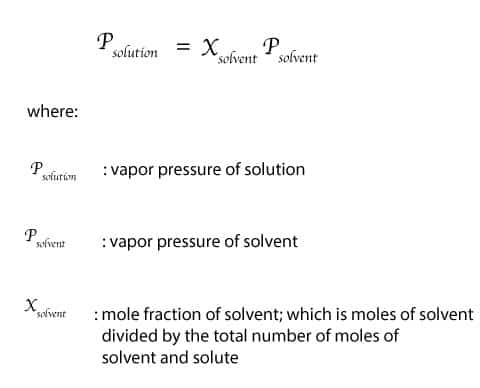
As you can see, once you know the mole fraction and vapor pressure of solvent, you can use Raoult’s law to predict the vapor pressure of solution. Solutions that obey Raoult’s law are called ideal solutions. But in real life we hardly find ideal solutions. As a result, let’s use the following illustration to further explore Raoult’s law. In the illustration, we will consider the rate of evaporation of pure water and salt water:
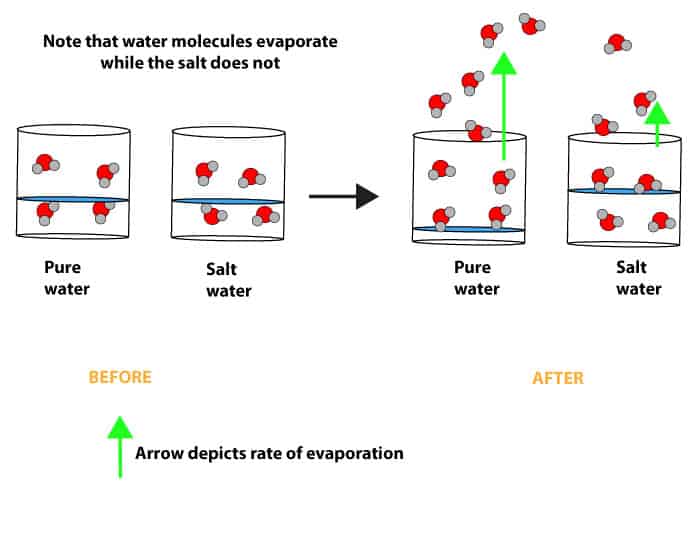
As you can see from the pictures above, the beakers on the left side of the arrow contain the same volume of liquid. However, one of them contains pure water, while the other contains salt water (solution of salt and water). If you repeat the experiment and observe these beakers overtime, you will notice that pure water evaporates faster than salt water.
Why is that? At least two molecular processes can explain what you saw. These molecular processes are:
- solvent-solvent interactions
- solute-solvent interactions
These molecular processes are shown in the following illustration:
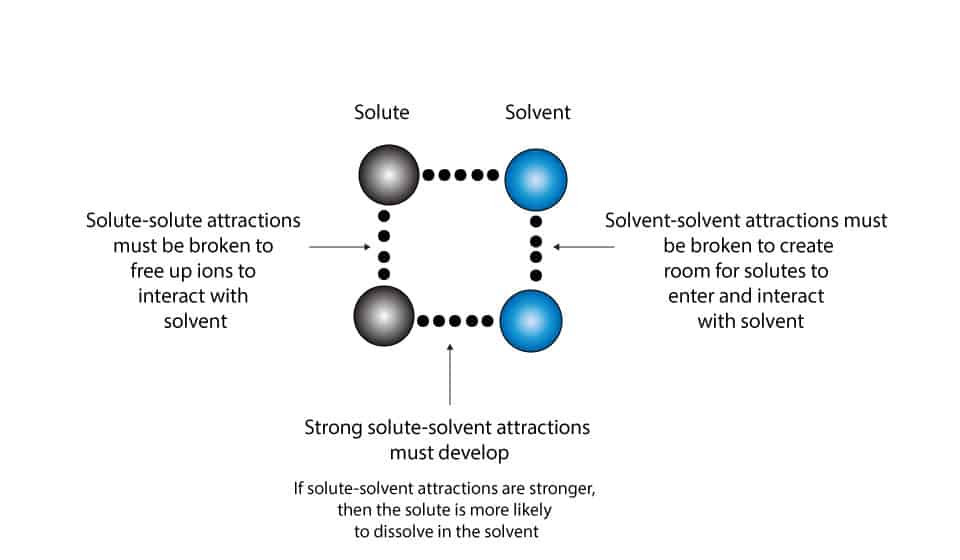
If the attractive forces between solute and solvent are stronger than the attractive forces between solvent and solvent, then only fewer solvent molecules will evaporate and turn into vapor.
In this case, salt water, where salt (NaCl) is the solute and water the solvent, the stronger attractive forces between sodium ions and water molecules and chloride ions and water molecules prevent many water molecules from turning into water vapor. Because of this, the vapor pressure of salt water will be less than that predicted by Raoult’s law. This is because more energy is required to break the attractive forces between the solute and solvent molecules.
On the other hand, if the attractive forces between solute-solvent are weaker than the attractive forces between solvent-solvent, then many solvent molecules will evaporate and turn into water vapor. Because of this, the vapor pressure of pure water will be greater than that predicted by Raoult’s law. This is because less energy is required to break the attractive forces between water molecules. In this case, in pure water, the attractive forces between water molecules is weaker than salt water. As a result, many water molecules will absorb the required energy and turn into water vapor.
In sum, properties of solutions that depend only on the concentration of particles (ions, molecules) in solution are called colligative properties. Examples of colligative properties include: vapor pressure lowering, boiling point elevation, and freezing point depression.