The oxidation state method is an “electron bookkeeping method” devised by chemists to help assign a number called oxidation number to an atom. This oxidation number tells us how many valence electrons an atom owns in a molecule or compound. By assigning and keeping track of this number, chemists can tell whether or not electrons are being transferred in a chemical reaction.
How do you determine the oxidation number of an atom in a molecule from scratch?
To determine the oxidation number of an atom in a molecule, you must start from the molecules’ electron dot diagram. In addition to that, you must apply the following rules to successfully use the molecules’ electron dot diagram to determine this number.
The first rule states that the:
- atom with lone pairs of electrons in an electron dot diagram fully owns the lone pairs
Let’s use the following electron dot diagram of water to illustrate this rule:
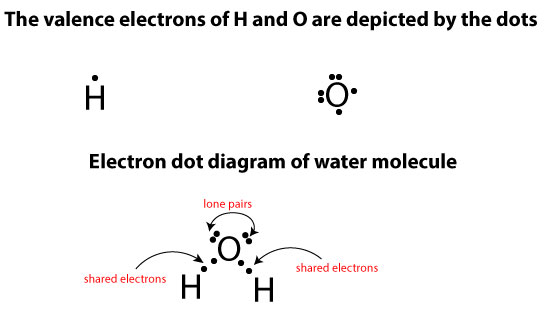
From the picture above, you can see that hydrogen (H) has only one valence electron (one dot on it), while oxygen (O) has six valence electrons. For hydrogen to become stable, it has to share its lone valence electron with another valence electron from oxygen. If the sharing happens as molecules of these atoms react to form water, hydrogen will achieve a duet (maximum of two electrons) while oxygen an octet (maximum of eight electrons). If you draw the electron dot diagram for one molecule of water, you will notice that oxygen has two lone pairs of electrons (unshared electrons) sitting on it, while hydrogen has none. You will also notice that each hydrogen atom shares its unpaired electron with another unpaired electron from oxygen. So, in a nutshell, what the first rule is saying is that the lone pairs of electrons sitting on oxygen belong to only and only oxygen.
The second rule states that the:
- more electronegative (EN) atom in a covalent bond owns all the shared electrons. As you recall, an electronegative atom is an atom that loves electrons so much that it will go out of its way to pull the shared electrons more towards itself.
From the periodic table, the electron hogs (most electronegative elements) are: fluorine (EN = 4.0), oxygen (EN = 3.5) chlorine (EN = 3.0), and nitrogen (EN = 3.0). From the list, you can see that oxygen is an electron hog, while hydrogen is not. Therefore, if we apply the second rule to the following electron dot diagram of water, you will see that oxygen gets all the shared electrons, while hydrogen gets nothing:
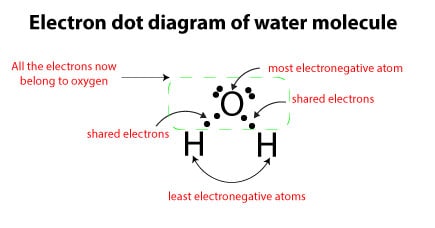
The third rule states that:
- if two atoms have the same electronegativity and are bonded to each other, then the shared electrons between them must be shared equally between them.
For instance, in a hydrogen molecule —where two atoms of hydrogen share their valence electrons to form a covalent bond — each hydrogen atom gets half of the shared electrons. See the following picture:
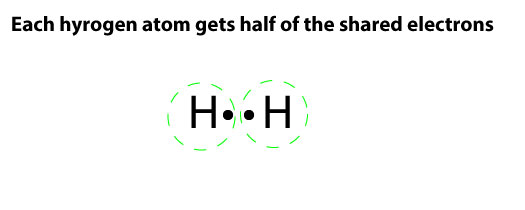
Now that we have illustrated these rules using the pictures above, let’s now apply them to determine the oxidation number of each atom in H2O. Again, here is its electron dot diagram:
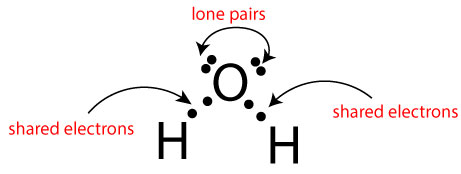
To do this, first, we will determine the valence electrons of H and O atoms in their free form (before they formed the chemical bond). As you may recall, H and O belong to the main group elements (representative elements). Because of this, the valence electrons of H and O will be equal to their group number. That is, since H belong to group one, it follows that the valance electrons of H will also be one. Similarly, since O belong to group six, it follows that the valence electrons of O will also be six.
Therefore, to get the oxidation number or state of an atom in a molecule, you must subtract the number of electrons assigned to it using the oxidation bookkeeping method from the number of valence electrons on the free atom. How do we assign this number to an atom in a molecule? To assign this number, we must follow the three rules discussed above. Here is how:

By applying rule 1, oxygen owns two lone pairs of electrons, while hydrogen owns zero. Together, that is 4 electrons for oxygen.
By applying rule 2, oxygen owns all the shared electrons, while hydrogen owns zero. Together that is another 4 electrons for oxygen. So, by the oxidation bookkeeping method, oxygen is assigned a total of 8 electrons, while hydrogen is assigned 0. If you now subtract the number of electrons assigned to H and O from their corresponding valence electrons, you will find that the oxidation state of hydrogen is +1 and oxygen, -2. Here is a table showing the calculation:
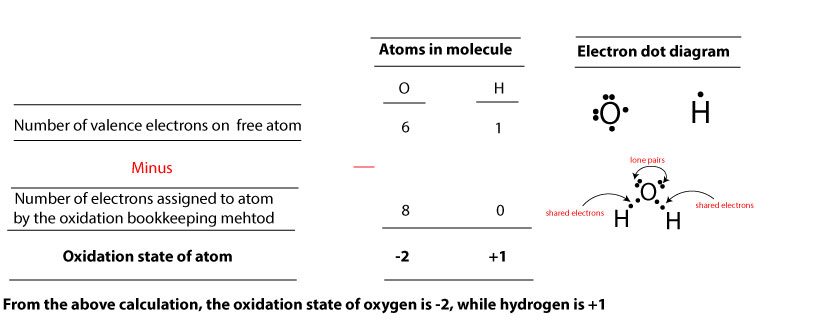
From the calculation, we can deduce that:
- if the oxidation number of an atom is negative, then it follows that the atom owns more electrons in the molecule or compound than it owns as a free atom.
- If the oxidation number of an atom is positive, then it follows that the atom owns more electrons as a free atom than it owns in the molecule or compound.
At this point, remember that the oxidation number or state of an atom is not the same as the charge of an atom. As you can tell, the oxidation number is a number derived from keeping track of electrons in a written balanced equation, while the charge is tied to the number of electrons lost or gained by an atom relative to the number of protons it has.
Also note that when writing the:
- oxidation number for an atom you must place the positive or negative sign before the number like so: +2.
- charge of an atom, you must place the positive or negative sign after the number like so: 2+
As you may have noticed, the oxidation bookkeeping method is a tedious way to assign oxidation numbers. Because of this, chemists have devised a shortcut for assigning oxidation states. To learn about this shortcut, click here.