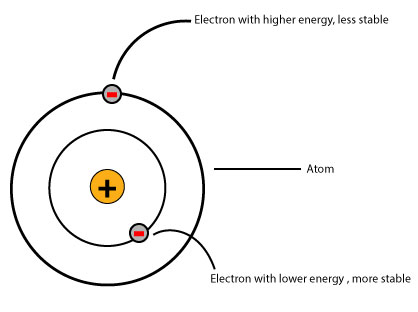
Factors that can affect the energy of an electron inside an atom include: distance, positive charge of the nucleus, electron-electron repulsion, and overlapping of atomic orbitals.
We know that the positive charge of the nucleus is as a result of the positively charged protons living inside the nucleus. Now how does this positive charge affect the energy of an electron? A way to think about this is to imagine that you have a north pole of a magnet attracted to the south pole of another. As you keep the south pole still while you move the north pole away from it, you will notice that the strength of the attractive force between them decreases with increasing distance. If you replace these magnets with stronger magnets, you will notice a stronger attractive force between them, but the strength of this force still decreases with increasing distance. If you relate these observations to the electrical attractions inside an atom, you can deduce that:
- when an electron is far away from the nucleus, the less it is attracted by the nucleus. And the less an electron is attracted by the nucleus, the higher its energy. And the higher its energy, the less stable the arrangement between the electron and the nucleus. The less stable the arrangement, the less stable the atom.
- when an electron is closer to the nucleus, then the stronger it will be attracted by the nucleus. And the stronger the nucleus attracts an electron, the lower the electron’s energy. And the lower the electron’s energy, the more stable the arrangement between the nucleus and the electron. The more stable the arrangement, the more stable the atom.
- when the positive charge of the nucleus is stronger, the stronger it will attract an electron closer to it.
Now, how does the inner electrons affect the attraction of the nucleus for the outer electrons in a multielectron atom? The inner electrons can neutralize some of the positive charge of the nucleus. This neutralizing effect called partial shielding can cause the outer electrons to be less strongly attracted by the nucleus. As a result, the outer electrons will have a higher energy. What about electron-electron repulsion, does these repulsions also affect an electron’s energy? Yes, they do. Recall that electrons carry a negative charge and charges of the same kind repel each other. If we put all these factors together, then we can say that if an atom has the following electron configuration: 1s22s22p1.Electrons in the 1s, 2s, and 2p orbitals will repel one another. As a result, electrons in the 2p orbital will have a higher energy than the electrons in the 2s orbital. In the same way, some of these atomic orbitals sometimes overlap one another. This overlapping can affect the energy of the electrons living in them.
Here is an illustration summarizing what we just learned: