To identify a redox reaction, we must first calculate the oxidation number of each atom in the reaction. If there is a change in oxidation number, then the reaction is a redox reaction. If there is no change in oxidation number, then the reaction is not a redox reaction. Now, let’s find out whether the following chemical equation represents a redox reaction:

First step: let’s assign oxidation numbers to the chemicals in the above equation. If we do, we will get the following equation:

As you can see, the oxidation number of Fe increases from 0 to +2, while the oxidation number of Cu2+decreases from +2 to 0. Since the oxidation numbers of the chemicals in the equation changes, then, we can confidently say that the equation represents a redox reaction.
Let’s try another. Does the following chemical equation represent a redox reaction:

First step: let’s assign oxidation numbers to the chemicals in the above equation. If we do, we will get the following equation:

Notice, the oxidation numbers of all the atoms in the equation remained the same before (reactant side) and after (product side) the reaction. Because of this, we can confidently say that the equation does not represent a redox reaction. Notice that on top of the hydrogen atom in (H2O), we see a total oxidation number of +2. This is because in water (H2O) we have two atoms of hydrogen and each atom of hydrogen has an oxidation number of +1. So, if we multiply the 2 by +1, we get a +2. The +2 is therefore the combined oxidation numbers of the hydrogen atoms in water.
Let’s try another. Does the following chemical equation represent a redox reaction:
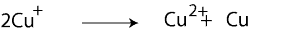
First step: let’s assign oxidation numbers to the chemicals in the above equation. If we do, we will get the following equation:
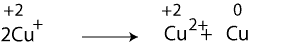
Notice that, Cu+ has an oxidation number of +1, but because there are two atoms of copper, the combined oxidation number is +2. Once you recognize that, you will notice that Cu+ is both oxidized to Cu2+and reduced to Cu. Because there is a change in oxidation number, we can confidently say that the above equation represents a redox reaction. Reactions in which only one chemical is both oxidized and reduced at the same time are called Disproportionation.
After reading this, you will realize that to identify redox reactions, we must master how to assign oxidation numbers to the chemicals in the equation. To learn more about oxidation numbers, click here.