A hydrate is a crystalline ionic compound that contains water in its structure. Hydrates are formed when ionic compounds dissolve in water and the resulting solution allowed to evaporate. As water in the solution evaporates, crystals of the compound form with some water bonded (attached) to the formula units inside the crystal. Examples of hydrates include: CuSO4.5H2O (bluestone), MgSO4.7H2O (Epsom salt), and Na2B4O7.10H2O (borax). The dot (.) you see in the hydrate’s chemical formula is a way to show that water molecules are bonded to each formula unit of the hydrate. For instance, in the hydrate MgSO4.7H2O, which is widely known as Epsom salt, the number, 7 in front of the H2O shows that for each formula unit of MgSO4(Magnesium sulfate) there are seven molecules of water bonded to it. Similarly, the number, 5 in CuSO4.5H2O shows that for each formula unit of CuSO4, there are five molecules of water bonded to it.
What happens when you heat a hydrate?
When you heat a hydrate, the water in it evaporates and leaves behind the crystalline compound. The crystalline compound is usually referred as anhydrous, meaning without water. While the evaporated water is called water of crystallization. The amount of water of crystallization is usually constant for the same compound but varies among different compounds. For instance, when you heat CuSO4.5H2O, the water in it evaporates and leaves behind anhydrous CuSO4. We can write an equation for the decomposition of CuSO4.5H2O by heat as:
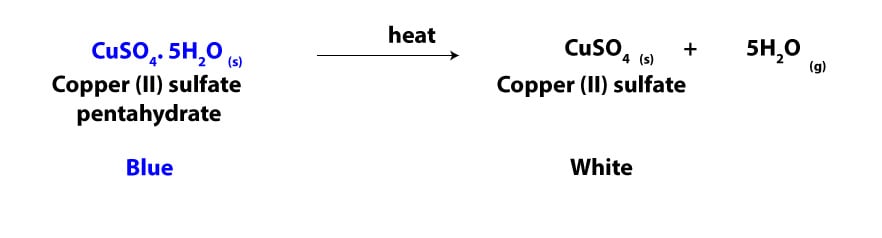
As you can see, the color of the hydrate CuSO4.5H2O is blue. When it is heated, the water in it evaporates and leaves behind anhydrous white solid, CuSO4. The hydrate CuSO4.5H2O is chemically called copper (II) sulfate pentahydrate. The roman numeral in parenthesis (II) represents the number, 2, and it specifies the oxidation state of the copper. While the Greek prefix, penta, specifies the number of water molecules in the hydrate. For the hydrate CuSO4.5H2O, this number is 5. As you may notice, naming a hydrate is similar to naming ionic compounds with some slight modifications.
What happens when anhydrous salt dissolves in water and solution allowed to evaporate?
When anhydrous CuSO4 dissolves in water and the resulting solution allowed to evaporate, some water molecules bond with CuSO4 to form the hydrate CuSO4.5H2O. We can write a chemical equation for this reaction as:

As you can see, the second reaction is the reverse of the first reaction (decomposition). Meaning the anhydrous compound, CuSO4 can react with water to get back the hydrate.
How do you calculate the percent of water in a hydrate?
To calculate the percent of water in a hydrate, you must recall the formula:
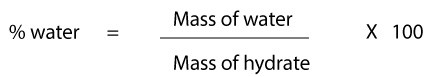
As you can see, the percent of water in hydrate is the ratio of mass of water to the mass of the hydrate all times 100
As an example, let’s solve the following question.
Calculate the percentage of water in bluestone, CuSO4.5H2O
To solve the problem, you will find the molar mass of the anhydrous salt, CuSO4 and molar mass of H2O. Using the periodic table, the molar mass of CuSO4 is 159.61 g/mol. And molar mass of water is 18.02 g/mol. Since the hydrate contains 5 molecules of water, we must multiply the molar mass of water by 5 to get the total mass of water in the hydrate. We will then add the mass of the water to the mass of anhydrous salt to get the total mass of the hydrate. Once we do that, we will then divide the total mass of water by the mass of the hydrate. Here is a setup of the calculation:
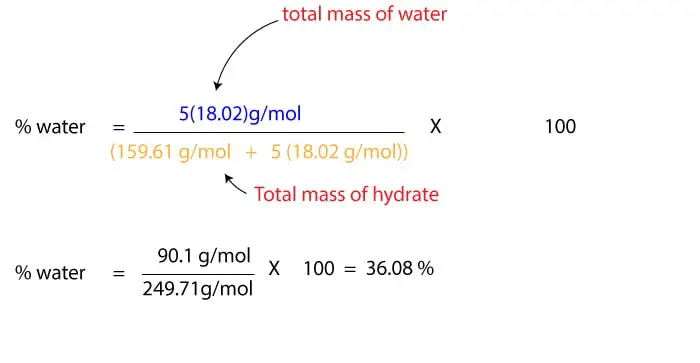
From the calculation, you can clearly see that the units of g/mol in the numerator and denominator cancel out. Hence the percentage composition of water in CuSO4.5H2O is 36.08 %.
Now, you try: calculate the percent of water in borax, Na2B4O7.10H2O. Ans: 47.24 %