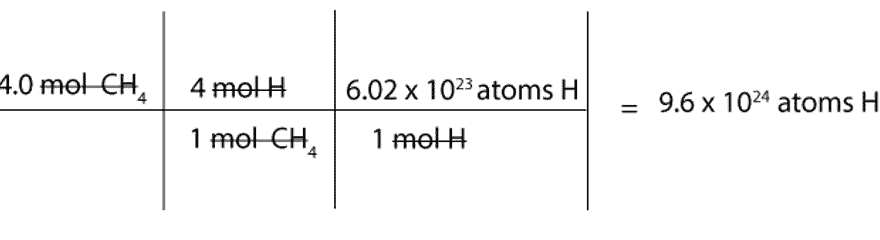To use a chemical formula to find the mole ratio of the atoms in it, we must first learn how to interpret the chemical formula in terms of moles. Once we do, we can then proceed to write ratio identities (conversion factors). We can write this ratio identities because the law of constant composition says that atoms combine in fixed mass ratio. Now, let’s further explain this using the chemical formula for methane: CH4
If we have a mole of CH4 molecules, we can interpret the formula, CH4 in terms of moles by saying that:
1 mol of CH4 contains 1 mol of carbon and contains 4 mol of hydrogen. If we replace the word contains with an equal sign, then we can write the following expression:
1 mol of CH4 = 1 mol of C = 4 mol of H
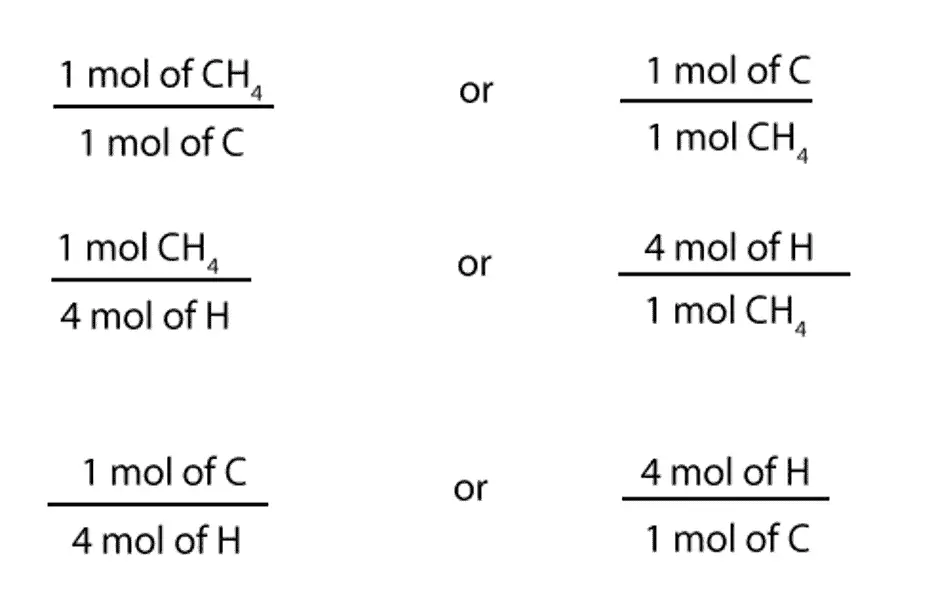
From the above expression, we can write the following mole-mole ratios:
1 mol of CH4 /1 mol of C or 1 mol of C / 1 mol of CH4
1 mol of CH4 / 4 mol of H or 4 mol of H / 1 mol of CH4
1 mol of C / 4 mol of H or 4 mol of H / 1 mol of C
Here is a much clearer way of representing the above ratios:
So, as you may have noticed, these are the ratio identities we will use to help convert between moles of one substance and moles of another. Now, let’s test our understanding by solving the following problem:
Question
How many atoms of H are there in 4.0 mol of CH4?
Strategy
We must first convert mol of CH4 to mol of H and then to atoms of H.
To convert from mol of CH4 to mol of H, we will use this mole-mole ratio as our conversion factor:
4 mol H / 1 mol CH4
To convert from mol of H to atoms of H, we will use the following conversion factor: 6.02 x 1023atoms H / 1 mol H. Recall that the constant 6.02 x 1023 is Avogadro’s number, and this number is usually used to convert from moles to atoms or molecules (particles) and vice versa.
Here is a complete set up of how to calculate the number of H atoms in 4 moles of methane:
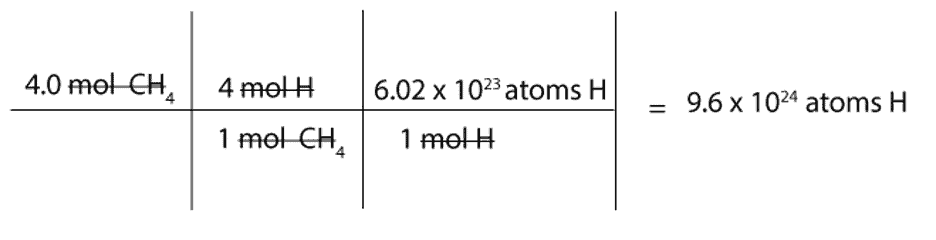
Another question
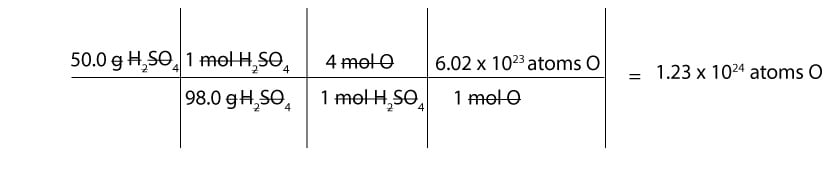
How many atoms of oxygen are present in 50 g of sulfuric acid (H2SO4)?
Strategy
Since we are given mass in grams, we must first convert from grams to moles and from moles to atoms.
- To convert from grams to moles of sulfuric acid, we must use the molar mass of sulfuric acid. And if you recall, the molar mass of sulfuric acid is 98.0 g
- To convert from moles of sulfuric acid to moles of O, we must figure out how many moles of O are in 1 mol of sulfuric acid. If you check the formula, H2SO4, you will notice that we have 4 mol of oxygen in 1 mol of sulfuric acid. Therefore, we can write the following mole-mole ratios:
4 mol O / 1 mol H2SO4 or 1 mol H2SO4 / 4 mol O
- To convert from moles of O to atoms of O, we must use Avogadro’s number. And recall that: 1 mol of any substance contains Avogadro’s number of atoms or molecules.
Here is a table showing how to calculate the number of O atoms in H2SO4:
Another question
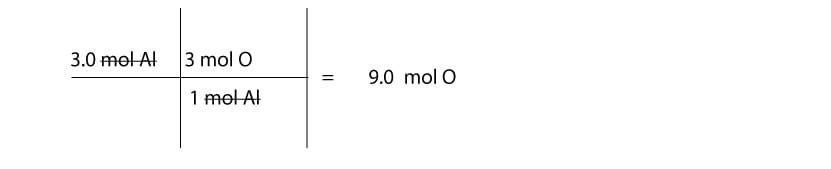
If there are 3 mol of aluminum (Al) in Al (OH)3, how many moles of oxygen (O) are in the sample?
Strategy
We must find the mole ratio between Al and O in Al (OH)3. If you multiply the atoms in parenthesis by the subscript, 3, you will notice that the mole ratio between Al and O is: 1 mol Al / 3 mol O or 3 mol O/1 mol Al. Since we want mol of O, we will use the mole ratio 3 mol O / 1 mol Al. Here is a table showing the calculation:
To learn how to use a chemical formula to go from molecules to atoms or grams of substance, click here