When asked to calculate the mass of water in grams when 4.50 g of methane (CH4) reacts with excess oxygen according to the equation:
CH4 + O2 –> CO2 + H2O
How should you proceed?
When asked to use the mass of a reactant and and a chemical equation to calculate the mass of another reactant or product. You should proceed as follows.
First, check the equation to ensure its balanced. If it’s not, you must balance it before using the equation to write mole-mole ratio.
If we apply the above principle to our equation: CH4 + O2 –> CO2 + H2O, you will notice that it’s not balanced.
To balance it, we will put a 2 in front of O2 and another 2 in front of H2O. Our balanced equation now appears: CH4 + 2O2 –> CO2 + 2H2O
Now, let’s interpret the equation in terms of moles so that we can write mole-mole ratio between methane and water. To do so, we will use the numbers in front of the chemicals as our moles. That is, we will say 1 mole of methane (CH4) reacts with 2 moles of oxygen (O2) to produce 1 mole of carbon dioxide (CO2) and 2 moles of water (H2O).
1 mol CH4 + 2 mol O2 –> 1 mol CO2 + 2mol H2O
The question says oxygen(O2) is in excess. This means that the other reactant, methane (CH4) is the limiting reactant, and the limiting reactant determines how much products (CO2 and H2O) the reaction can make. So, to calculate the mass of water produced, we must first calculate the moles of H2O that will react with the 4.50 g of methane.
To do so, we will divide 4.50 g methane by its molar mass (16.05 g/mol) to get moles. Once we get moles of methane, we will use the mole ratio between methane and water in the chemical equation to convert from moles of methane to moles of water. Once we get moles of water, we will multiply moles of water by its molar mass to convert moles of water to grams of water.
Here is an illustration of the steps, we just discussed

Here is a summary of the calculation:
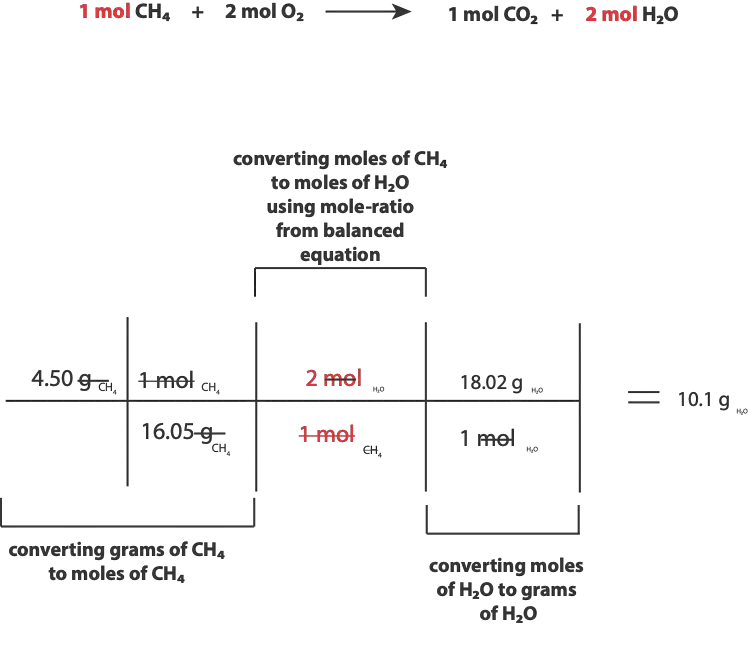
As you can see, the mass of water produced is 10.1 g put to 3 significant figures. The calculation shown in the table can be applied to other similar stoichiometry problems.
To learn how to use a chemical equation to convert from moles of one chemical to moles of another, click here
To learn how to determine the limiting reactant in a chemical reaction, click here.