What’s Molarity?
Molarity, with symbol M, is defined as the number of moles of solute present in a liter of solution. Mathematically, Molarity can be expressed as: Molarity, M = moles of solute/Liter of solution.
Notice, volume of solution is in liters. If volume is not given in liters, you must convert to liters before you calculate solution concentration. Similarly, if mass of solute is given, you must convert mass to moles before you calculate solution concentration. Molarity is a derived unit (moles/Liter). Therefore, you can use it as conversion factor to convert from moles to liters and vice versa. Chemists like reporting concentration in Molarity because it ties well with the mole concept and can be used to determine the number of ions or molecules in a solution.
For example,
You made a 200mL solution of glucose
that contains 0.3 mol of glucose. What’s the
solution concentration?
Answer
First, we must convert the 200 mL to liters: Since 1000 mL = 1 L
We can write that, 200 mL x 1L/1000 mL = .20 L
Therefore, Molarity = mol/L = .3 mol glucose/.20L = 1.5 mol/L = 1.5 M glucose.
Now, if we modify the above example by changing the moles of glucose to grams of glucose, we will get the following question:
You made a 200mL solution of glucose
that contains 54 g of glucose. What’s the
solution concentration in Molarity?
To solve this question, we will recall that: Molarity (M) = moles of solute/Liter of solution
But since we are given grams of glucose not moles of glucose, then it follows that we must convert the 54 g of glucose into moles of glucose. To do this, we will need the molar mass of glucose. But to calculate molar mass, we will need the chemical formula of glucose. This formula is : C6H12O6. Next, we will then look on the periodic table for the molar mass of each atom, and then multiply this value by the corresponding number of each atom in the formula.
If we do, we will get:
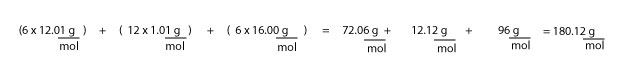
Therefore, the molar mass of glucose is 180.12 g/mol. Next, to get the moles of glucose, we must multiply 54 g by the inverse of 180.12 g/mol, which is— 1 mol/180.12 g— to get our answer in moles. If we do, we will get:
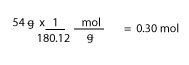
Now, just like the previous example, we must divide moles of glucose by the volume of the solution in liters to get Molarity. If we do, we will get:
Molarity of glucose = 0.30/0.20L = 1.5 M
As you can see, this answer is the same as the answer we got in the previous example. Therefore, regardless of whether you are starting from mass or moles of a particular chemical, you will always arrive at the same answer.
To learn how to prepare a chemical solution from scratch, click here.
To learn how to calculate solution concentration in in mass percent, click here