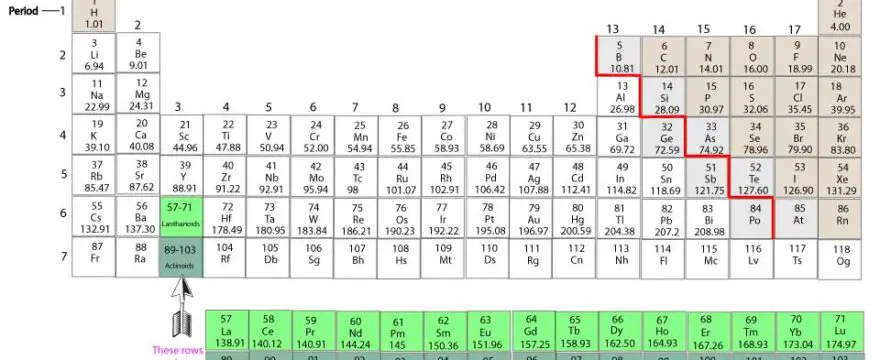
Valence electrons are the electrons in the outermost shell of an atom. These electrons are easily accessible and used by atoms to bond with one another. These bonds usually form when atoms lose, gain, or share valence electrons.
With few exceptions and slight modification, we can use the group numbers on the periodic table to determine the number of valence electrons of an element.
Exceptions— elements in group 10 have 10 valence electrons while helium (He) in group 18 has 2 valence electrons
Modification — elements in group 11 to 18 have valence electrons equal to the last digit of the column number(group number). That is elements in group 11 have 1 valence electrons, group 12, 2 valence electrons and group 18, 8 valence electrons, except for He, which has 2.
Here is an illustration of the periodic table
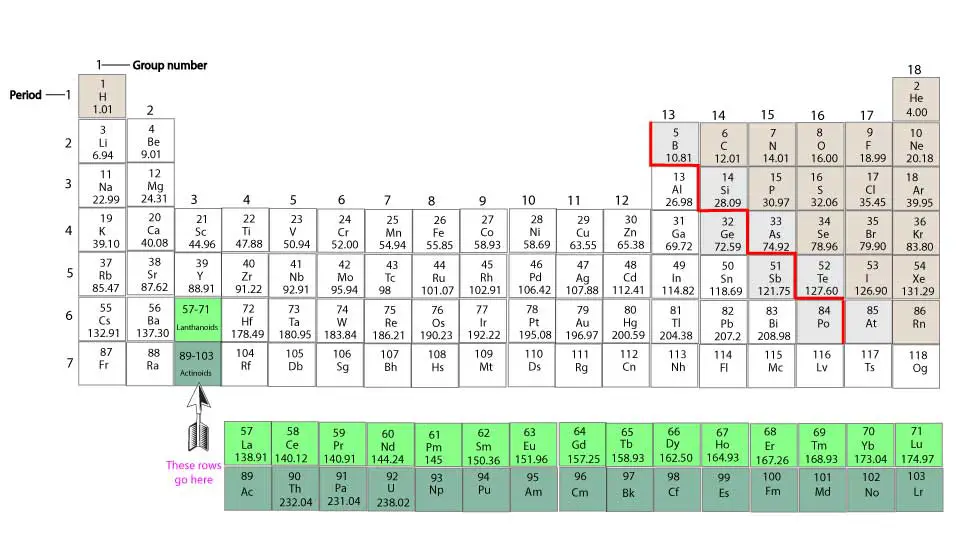
From the periodic table, the vertical columns numbered from 1 to 18 are called group numbers. Elements in the same group have similar chemical properties. Elements in single digit group numbers that is from 1 through 9, have total valence electrons equal to the group number.
For example, Hydrogen (H) and the rest of the elements in group 1 have one valence electron each.
Beryllium (Be) and rest of elements in group 2 have 2 valence electrons each
Scandium (Sc) and rest of elements in group 3 have 3 valence electrons each
Titanium (Ti) and rest of elements in group 4 have 4 valence electrons each
Vanadium(V) and rest of elements in group 5 have 5 valence electrons each
Chromium (Cr) and rest of elements in group 6 have 6 valence electrons each
Manganese (Mn) and rest of elements in group 7 have 7 valence electrons each
Iron (Fe) and rest of elements in group 8 have 8 valence electrons each
Cobalt (Co) and rest of elements in group 9 have 9 valence electrons each
Exception
Nickel (Ni) and rest of elements in group 10 have 10 valence electrons each
Modification
Elements in group 11 to 18 have valence electrons equal to the last digit of the column number
Copper (Cu) and rest of elements in group 11 have 1 valence electrons each
Zinc (Zn) and rest of elements in group 12 have 2 valence electrons each
Boron (B) and rest of elements in group 13 have 3 valence electrons each
Carbon (C) and rest of elements in group 14 have 4 valence electrons each
Nitrogen (N) and rest of elements in group 15 have 5 valence electrons each
Oxygen (O) and rest of elements in group 16 have 6 valence electrons each
Fluorine (F) and rest of elements in group 17 have 7 valence electrons each
In group 18, only Helium (He) has 2 valence electrons, while the rest have 8 valence electrons. He is placed in group 18 because its chemical behavior is like the other elements in group 18
As you can see, once you remember the few exceptions, you can pretty much use the group number to identify an element’s valence electrons.