Molality (m) is the moles of solute dissolved in kg of solvent. While Molarity(M) is the moles of solute dissolved in volume of solution in Liters.
Mathematically, Molality is expressed as:

While Molarity is expressed as:

As you can see, the numerator—moles of solute— is the same for both Molality and Molarity but the denominator is different for Molality. Why is that? Generally, as temperature rises the average kinetic energy of molecules also rises and as the kinetic energy rises molecules move (vibrate, rotate and translate) a lot more, causing the distance between the solution molecules to increase. And as the distance increase the volume of the solution slightly increase.
Likewise, as the temperature drops, the average kinetic energy of molecules also decrease, and as the kinetic energy decreases molecules move (vibrate, rotate and translate) a lot less, causing the distance between the solution molecules to decrease. And as the distance decrease, the volume of the solution slightly decrease. Because the volume of solution can change depending on temperature, Molarity is not an accurate measure of solution concentration.
As a result, Chemists sometimes prefer to calculate solution concentration in Molality (m). Because with Molality you divide the moles of solute by the mass of solvent. And mass does not increase or decrease depending on temperature. As a result, Molality is an accurate measure of concentration.
Now, let’s solve the following problem on Molality
You prepared a solution by adding 5.00 g of sodium chloride (NaCl) in 100 g of water. Calculate the Molality of the solution.
To start, recall that Molality (m) is moles of solute divided by Kg of solvent. However, we are given only the mass of the solute (NaCl). This means we must convert mass of NaCl to moles of NaCl. To convert, we divide grams of solute by molar mass of solute to get moles. Once we get moles, we divide it by mass of solvent in kg. However, the mass of solvent (water, H2O) is given in grams. Therefore, we must convert mass of solvent to kg before we divide. To convert, we recall that 1000 g is equal to 1 kg. Here is a summary of the calculation, that is Molality (m):
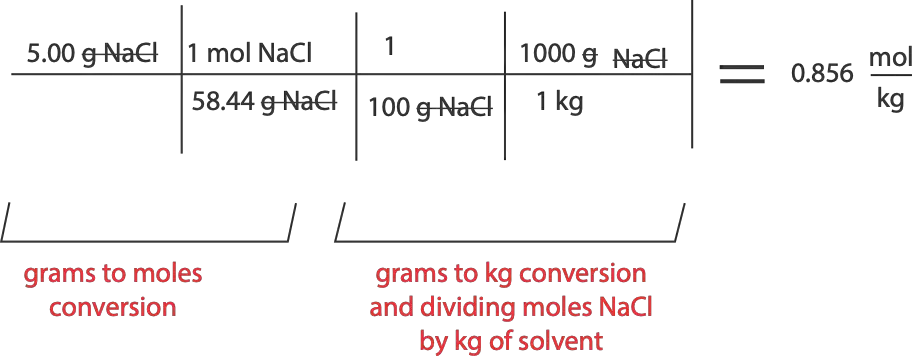
Hence, the Molality (m) of the solution is 0.856 mol/kg.
To learn how to calculate solution concentration in Molarity, click here