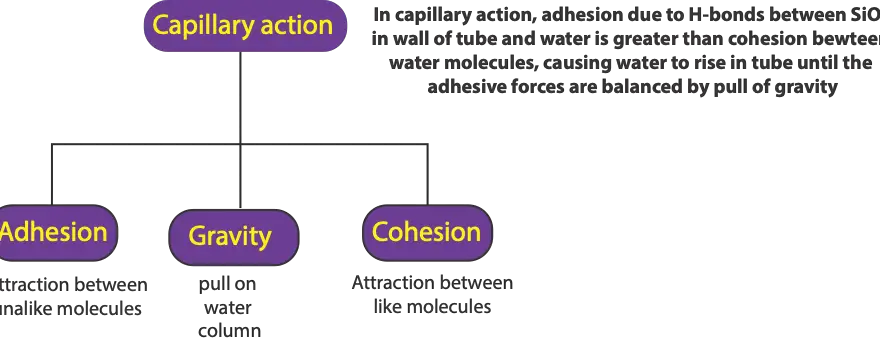
Capillary action is when water rises in a small glass tube because of the attraction between molecules of silicon dioxide (SiO2) in walls of glass tube and water (H2O) molecules. This attraction called hydrogen – bonds forms when hydrogen in water bonds with the oxygen in silicon dioxide. These hydrogen- bonds between water and walls of tube are much stronger than the hydrogen bonds present between the water molecules. As a result, the water rises in the tube until the attractive force is balanced by the pull of gravity.
As you can tell from the above statement, three types of forces are responsible for capillary action. First is adhesion, second is cohesion, and third is gravity.
Adhesion is the attraction between dissimilar molecules. In our situation, that is the attraction between molecules of water and molecules of silicon dioxide in the walls of the glass tube
Cohesion is the attraction between identical molecules. In our situation, that is the attraction between water molecules.
Gravity is the force that pulls matter toward the earth.
In summary, we will apply these three forces by saying that in capillary action, the adhesion between walls of tube and molecules of water is greater than the cohesion between water molecules. This causes water to rise in tube until the adhesive forces are balanced by the pull of gravity.
Here is an illustration summarizing the three types of forces responsible for capillary action

Capillary action is crucial to plants survival. Plants absorb nutrients from the soil and transport to all parts against the force of gravity.