Osmosis is the movement of solvent particles through a semipermeable membrane from a region of higher solvent concentration (lower solute concentration) to a region of lower solvent concentration (higher solute concentration).
Osmotic pressure is the pressure applied to the solution to stop osmosis by preventing solvent molecules from moving from the pure solvent side across the semipermeable membrane into the solution. The more concentrated the solution is the greater the pressure required to stop it.
As a result, osmotic pressure is directly proportional to the molarity of the solution, c.
Mathematically, osmotic pressure is expressed as:
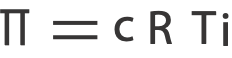
where,
c is the concentration in molarity
R is the universal gas constant with a value of 0.0821 L atm/mol K
T is the temperature in Kelvin and,
i is the number of particles per formula unit of solute
What is a semipermeable membrane?
A semipermeable membrane is a material with tiny pores that allows only certain kinds of molecules or ions to pass through it. Examples of semipermeable membranes include cell membranes in plants and animals and cellophane, a polymer made from cellulose. Osmosis is necessary in biological systems. In humans, osmosis helps transport fluids and solutes out of the kidneys and through cell membranes, and in plants, osmosis helps transport nutrients through plant roots up to their leaves.
How to calculate molar mass from osmotic pressure
Here is question:
The osmotic pressure of a solution of 5.0g of a molecular substance in 1.0 L of water was 1.8 x 10-3 atm at 25ºC. Calculate the molar mass of the molecular substance.
Strategy
From the question, we are given:
Mass of substance = 5.0 g
Volume of water = 1 L
Osmotic pressure () = 1.8 x 10-3 atm
Temperature (T) = 25ºC, but when converted to Kelvin, we get: 25ºC + 273 = 298 K
Gas constant, R = 0.0821 L atm/mol K
Because the substance is molecular, the number of particles of solute (i) is = 1
From the osmotic pressure equation, we have
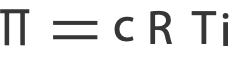
And since we know all the variables in the equation but concentration in Molarity, c, we isolate c and solve for it.

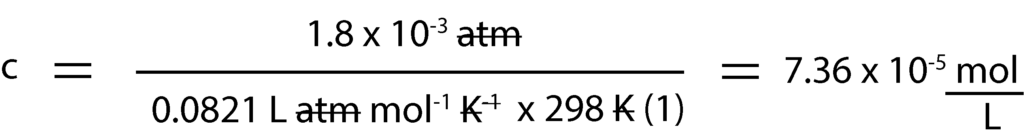
Next, we convert concentration in Molarity, c to moles by multiplying Molarity by volume of volume of water, 1L. That is:
Lx 7.8 x 10-5 mol/L= 7.8 x 10-5 mol
Next, since mass (m) = moles (n) x Molar mass (Mm), and we know mass and moles of substance. It follows that to get Molar mass (Mm) of substance, we divide mass of substance by its moles to get its molar mass.
That is Molar mass of substance = mass (g)/moles, and once we plug in the values, will get
5.0 g/7.8 x 10-5 mol = 6.8 x 104 g/mol
To learn about other colligative properties click here