Chemical reactions occur because chemicals want to be stable. For a reaction to occur spontaneously, the products of the reaction must have lower energy than the reactants. Lower energy means that as the reaction forms more stable products, the potential energy of the products decreases, releasing the excess energy to the surroundings. In chemistry, a spontaneous reaction is one that once started can occur on its own without continuous supply of energy from outside the reaction. To illustrate, consider the following model:

When the stopcock is opened, some of the gas molecules in bulb A travels spontaneously into the evacuated bulb B and fills it up. The reverse process of gas molecules compressing back into bulb A is non-spontaneous. Another spontaneous process is when oxygen reacts with hydrogen to form water:

The forward reaction, which forms water is spontaneous, hence product favored. In contrast, the reverse reaction, in which water decomposes to produce oxygen and hydrogen is non-spontaneous. For the reverse reaction to occur, we must intervene by continuously supplying the reaction with energy.
Two factors determine the spontaneity of a chemical or physical change. First is the release or absorption of energy at constant pressure, called enthalpy change (∆H). Second is the decrease or increase in molecular disorder, called entropy (∆S). Both factors must be considered when deciding the spontaneity of a process.
A spontaneous process is favored by a negative heat of reaction (negative – ∆H, exothermic) and an increase in entropy (positive +∆S)
A non-spontaneous process is favored by an increase in heat of reaction (positive ∆H) and a decrease in entropy (negative -∆S)
Exception, it’s possible for an endothermic process (positive +∆H) to be spontaneous because of its high entropy (positive +∆S). Example, melting of ice [∆H° = +6.01 kJ/mol; ∆S° = +22.0 J/K.mol]. And the reaction of barium hydroxide octahydrate with ammonium chloride [∆H° = +80.3 kJ/mol; ∆S° = +428.0 J/K.mol]. Here is the equation of the reaction:
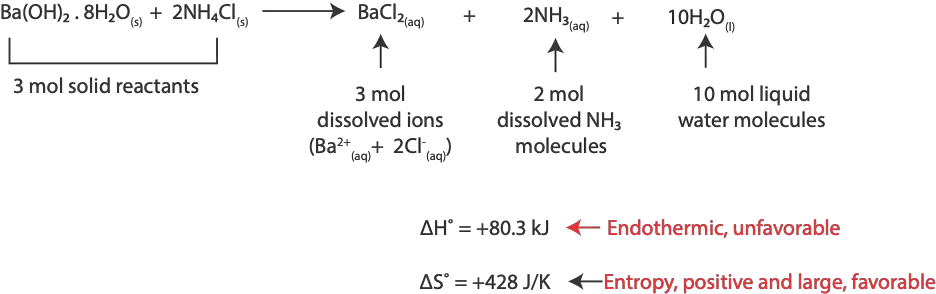
As you noticed, entropy is large and positive, because 3 moles of solid reactants produced 3 moles of dissolved barium and chloride ions, 2 moles of dissolved ammonia molecules, and 10 moles of liquid water molecules.
In contrast, it’s possible for an exothermic process (negative – ∆H) to be non-spontaneous because of its low entropy. Example, the conversion of liquid water to ice above 0°C is favored by enthalpy [∆H° = -6.01 kJ/mol], but its non-spontaneous because entropy is negative [∆S° = -22.0 J/K.mol].
How do you decide whether a reaction is spontaneous?
We must take both factors into account and define a new quantity called Gibbs free energy change (∆G) to help us predict whether a reaction will be spontaneous. Mathematically, Gibbs free energy (∆G) is expressed as: ∆G = ∆H – T∆S, where each symbol means—-
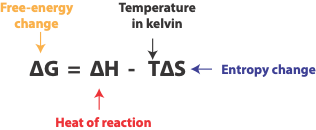
The value of the free-energy change, ∆G determines whether a chemical or physical process will be spontaneous.
If ∆G is negative, meaning ∆G < 0, the process is spontaneous
If ∆G is positive, meaning ∆G > 0, the process is non-spontaneous
If ∆G = 0 the process is at equilibrium
Because the term T∆S is temperature dependent, it’s likely that some processes might be either spontaneous or non-spontaneous at high or low temperature.
At low temperature, for example, a positive ∆H (unfavorable) term might be larger than a positive T∆S (favorable) term, resulting in a non-spontaneous process. At higher temperature, however, the T∆S might be larger than the positive +∆H. As a result, an endothermic process that is otherwise non-spontaneous at low temperature can become spontaneous at higher temperature. This is exactly what happens when ice is transitioning to water. At a temperature below 0°C, the melting of ice is non-spontaneous because the ∆H term is larger than the T∆S term, resulting in positive ∆G. However, at a temperature above 0°C, the melting of ice is spontaneous, because the T∆S term is larger than the ∆H term, resulting in negative ∆G. At exactly 0°C, the two terms are balanced. Here are the calculations to support the explanations:
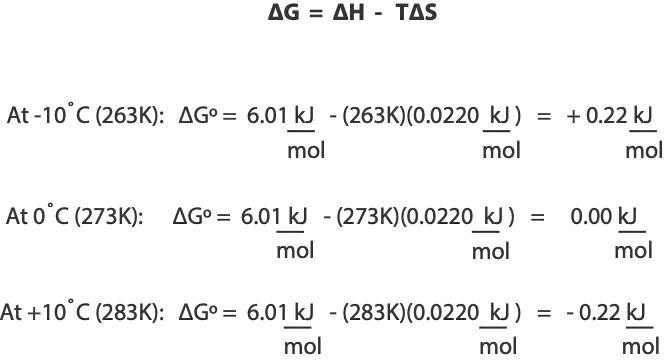
Notice that temperature in the Gibbs’ equation is in kelvin, as a result, you must convert temperature from Celsius to Kelvin. Also, the heat of reaction for melting ice is ∆H° = +6.01 kJ/mol, and its entropy ∆S° = +22.0 J/K mol. Since 1000 J = 1 kJ, you must divide entropy in J by 1000 to convert J to kJ so that you can have both ∆S° and ∆H° in the same units.
To learn why some reactions release energy to the surroundings while others absorb energy from the surroundings, click here