Solubility is the maximum amount of solute that will dissolve in a specific amount of solvent at a particular temperature and pressure. Solubility is usually expressed in grams of solute dissolved in 100 grams of solvent. And solubility can be controlled by temperature and pressure. Depending on the amount of dissolved solute, solutions can be classified as:
- Saturated
- Unsaturated
- Supersaturated
What’s saturated solution?
A saturated solution contains the maximum amount of dissolved solute in a certain amount of solvent at a particular temperature and pressure. When a solution is saturated, there is a dynamic equilibrium between undissolved and dissolved solute. That is some solvent molecules attract solute molecules and pulls them into solution. While some dissolved solutes recrystallize out of solution to form solute molecules. When dissolving and recrystallizing of solute happen at the same rate, a saturated solution is in equilibrium with its solute and solvent. Here is an illustration of the dynamic equilibrium.

What’s unsaturated solution?
Unsaturated solution contains less amount of dissolved solute in a certain amount of solvent at a particular temperature and pressure. That is solute concentration in unsaturated solution is less than its solubility. As a result, more solute can be added until the solution becomes saturated
What’s supersaturated solution?
Supersaturated solution contains more than the maximum amount of dissolved solute in a certain amount of solvent at a particular temperature and pressure. That is supersaturated solution contain more than the equilibrium concentration of solute at a certain temperature and pressure. To make a supersaturated solution, a saturated solution is made at a higher temperature and then slowly cooled to a lower temperature. Slow cooling is necessary to keep the excess dissolved solute in solution at a lower temperature. Because of the excess solute, supersaturated solution is unstable, and the excess solute will crystallize when a small amount of solute crystal, called seed crystal is added. Crystals can also form when inside the beaker is scratched with spatula or stirring rod.
Here is a graph of solute solubility showing the three types of solutions in different regions of the graph
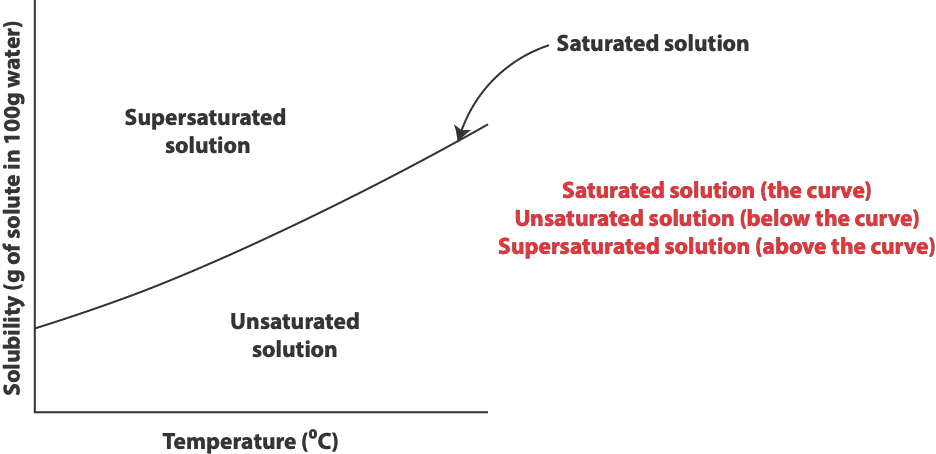
As you noticed, saturated solution is depicted by the smooth curve, unsaturated solution below the curve and supersaturated solution above the curve.
What key factors affect solubility?
The key factors include:
- Temperature
- Pressure
What’s the effect of temperature on solubility of solids?
Generally, if the enthalpy of solution is positive (endothermic), the solubility of many compounds increases as the temperature increase. The following graph shows the solubility of some ionic compounds at various temperatures.
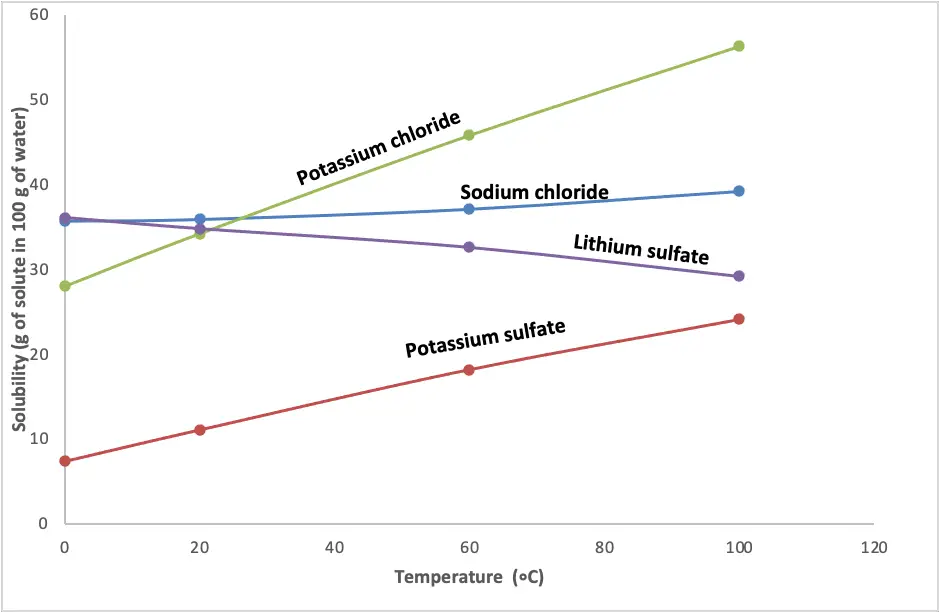
As you can see, at the same temperature, some compounds are more soluble than others. Why is that? One reason is the strength of the attractive forces between the ions in the compound (lattice energy). If the bond is weak, then less energy is put in to break it. If the bond is strong, then more energy is put in to break it. However, for lithium sulfate (L2SO4), its solubility decreases with increasing temperature.
Why is that? When lithium sulfate dissolves in water, it releases energy to the surroundings (exothermic). So, adding energy by increasing the temperature inhibits L2SO4 releasing energy during dissolving, hence decreasing its solubility. As s a result, solubility decreases with temperature if the enthalpy of solution is negative (exothermic).
What’s the effect of temperature on solubility of gases?
Solubility of gases decrease as temperature increase. This is because as temperature increase the average kinetic energy of gas molecules increase. As the energy of these gas molecules increase, they gain enough energy to overcome the attractive forces of the solvent molecules. And as they escape from solution, their solubility decreases. Therefore, when can of cold soda is left opened and placed on a countertop at room temperature, it will taste flat after some time.
What’s the effect of pressure on solids solubility?
In solids, the molecules or ions are closely packed. As a result, no amount of pressure can force these molecules or ions into solution. Therefore, pressure has no effect on solids solubility.
What’s the effect of pressure on solubility of gases?
Because gas molecules have lots of empty spaces between them, increasing the pressure concentrate the molecules and force them to collide with surface of solvent. And as they collide with surface, more gas molecules go into solution, increasing their solubility. This is the reason why when you open a can of soda, you suddenly see bubbles of carbon dioxide molecules gushing out of the container. This fizzing occurs because carbon dioxide is pressurized into solution by applying pressure greater than standard atmospheric pressure (1 am = 760 mm Hg).
The relationship between gas pressure and solubility is known as Henry’s law. Mathematically, Henry’s law is expressed as:
Sgas = kH x Pgas
where:
- Sgas is the solubility of the gas in mol/L
- kH is the Henry’s law constant and is specific for each gas-solvent combination at a given temperature. It has units of mol/L atm
- Pgas is the pressure of gas in atm.
Question
The partial pressure of CO2 gas inside a can of soda is 5.0 atm at 25°C. Calculate its solubility. Henry’s constant for CO2 dissolved in water = 3.3 x 10-2 mol/L atm at 25°C.
Solution
To solve this problem, we must recall Henry’s equation: Sgas = kH x Pgas
From the question, we know
kH = 3.3 x 10-2 mol/L
Pgas = 5 atm
Next, we plug the values into the equation and solve for solubility of carbon dioxide gas.
That is,
Sco2 = 3.3 x 10-2 mol/L. atm x 5.0 atm = 0.165 mol/L = 0.17 mol/L (Rounded to 2 significant digits)