Mole fraction is another way of expressing solution concentration. Mole fraction is the ratio of moles of solute to the total number of moles of solute and solvent.
The symbol, X with a subscript is normally used to denote the mole fraction of solute or solvent. That is XA is the mole fraction of solute A, and XB is the mole fraction of solvent B.
For instance, if an aqueous solution consists of solute A and solvent B. We can express the mole fraction of solute A as:

And the mole fraction of solvent B as:
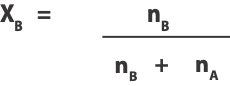
Where nA is the moles of solute A and nB moles of solvent B.
Since each component in solution contributes a fraction of the total moles, the sum of all component’s mole fraction must equal to 1.
The mole percent is the mole fraction expressed as a percentage.
That is Mole percent (mol %) = mole fraction x 100
Mole fraction gives the actual proportion of solute or solvent particles in the solution.
Question
Calculate the mole fraction of NaCl in an aqueous solution that contains 20.0% NaCl by mass.
Solution
Assume 100 g of solution and convert mass in percent to mass in grams
Since solution is aqueous, it means the solution consists of only NaCl and water. And since concentration of NaCl in solution is in mass percent, we must assume 100 g of solution, and then convert the 20.0% NaCl to 20.0 g NaCl. Once we do, we will then subtract the mass of NaCl from the 100 g of solution to get mass of water in g. That is
mass of solvent (H2O) in grams = 100 g solution – 20.0 g NaCl = 80.0 g H2O.
Convert mass of each component to moles
For molar mass NaCl, will calculate as:
{(1 mol x 22.99 g/mol) + (1 mol x 35.45 g/mol)} = 58.44 g/mol
For moles of NaCl, will calculate as:
20.0 g x 1 mol/58.44 g = 0.342 mol NaCl
For molar mass H2O, will calculate as:
{(2 mol x 1.01 g/mol) + (1 mol x 16.00 g/mol) =18.02 g/mol
For moles of H2O, will calculate as:
80.0 g x 1 mol/18.02 g = 4.44 mol
Total moles of solute (NaCl) and solvent (H2O) = 0.342 mol + 4.44 mol = 4.78 mol
Mole fraction of NaCl (XNaCl) = mol of NaCl/ moles of (solute and solvent)
Mole fraction of NaCl (XNaCl) = 0.342mol/4.78mol = 0.0715
To learn how to calculate solution concentration in Molarity, click here and in mass percent, click here.