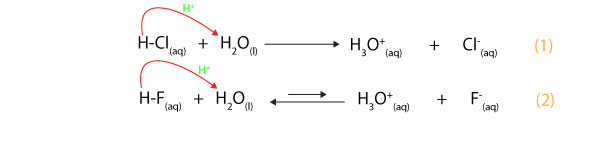
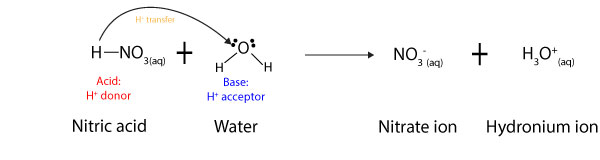
Is conjugate acid always stronger than its conjugate base? No! According to Brønsted-Lowry the strength of an acid depends on how well it can donate a proton, while the strength of a base depends …
Continue Reading about Is conjugate acid always stronger than its conjugate base? →