From the video, you will learn how to solve the following question: Copper has two isotopes. Copper – 63 has an atomic mass of 62.93 amu and an abundance of 69.17%. What is the atomic mass of the …
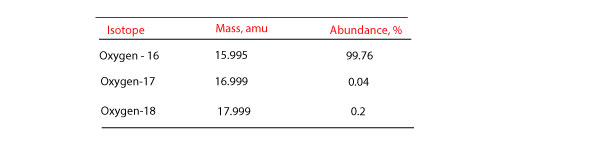
How do you calculate atomic mass of an element from isotopic mass and percentage abundance?
How do you calculate atomic mass? To calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom. To accomplish this, we usually use an …