What's Bohr's atomic model? …
What’s Rutherford’s atomic theory and what questions did critics ask about his theory?
What's Rutherford atomic model? Rutherford's atomic model consists of a nucleus. This nucleus contains positively charged particles (pluses) called protons. Years later, a second particle, called the …
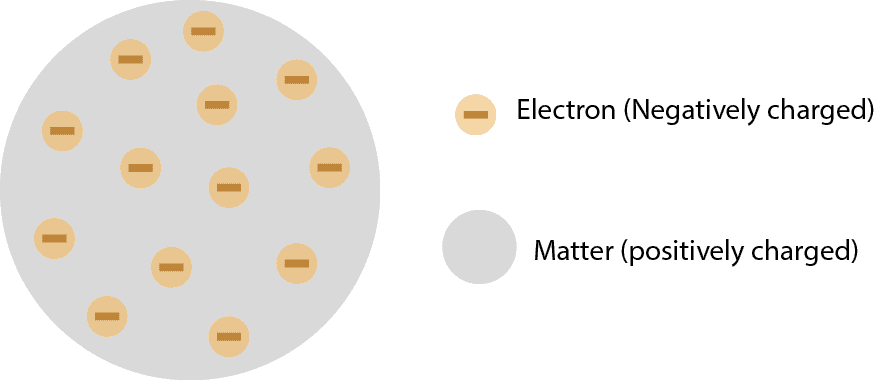
What’s Thomson’s atomic theory and what questions did critics ask about his theory?
What's Thomson's atomic theory? To determine whether atoms really consist of other particles, a scientist called J. J. Thomson carried out the now famous cathode-ray tube experiment from which he …
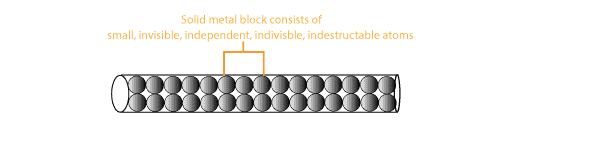
What’s Dalton’s atomic theory and what questions did critics ask about his theory?
What’s Dalton’s Atomic theory? Dalton's atomic theory consists of a series of suggestions called postulates. These postulates are: All elements consist of small independent, indestructible and …
How the law of constant composition, conservation of mass, and multiple proportions led to the atomic theory
What's the atomic theory? The atomic theory is based on the finding that all matter consists of invisible particles called atoms. What laws help develop the Atomic theory? These laws are: Law of …
What’s an Atom and why is it called so?
What's an Atom? An atom: is the smallest particle of an element that can exist has the element's chemical properties and, can take part in a chemical reaction. …
Continue Reading about What’s an Atom and why is it called so? →