From the video, you will learn how to solve the following question: Copper has two isotopes. Copper – 63 has an atomic mass of 62.93 amu and an abundance of 69.17%. What is the atomic mass of the …
How to calculate percentage abundance using atomic and isotopic masses
To calculate percentage abundance, you must recall the atomic mass of an element is calculated by using the formula: In the above formula you see fractional abundance. How do you get that? …
Continue Reading about How to calculate percentage abundance using atomic and isotopic masses →
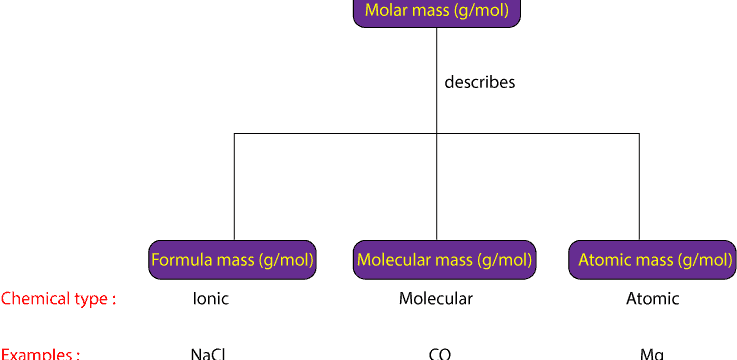
Molar mass: What is it and how to calculate the molar mass of hydrate?
What is molar mass and how do you calculate it? Molar mass is the general term used to describe the mass in grams of one mole (mol) of a chemical substance. Usually, molar mass has units of grams …
Continue Reading about Molar mass: What is it and how to calculate the molar mass of hydrate? →
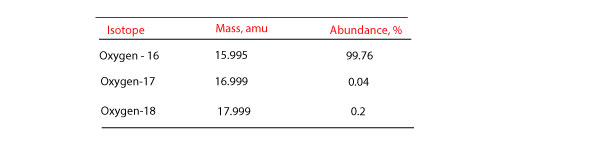
How do you calculate atomic mass of an element from isotopic mass and percentage abundance?
How do you calculate atomic mass? To calculate the atomic mass of an element, we have to calculate how much each isotope contributes to the mass of the atom. To accomplish this, we usually use an …
What’s atomic mass and why is it that it has no units?
What’s atomic mass? Atomic mass is the mass of an atom. However, when you look on the periodic table, you usually don’t see units attached to atomic mass. Why are atomic masses unitless? We can’t …
Continue Reading about What’s atomic mass and why is it that it has no units? →