How to convert from mole to number of molecules Since 1 mole of any substance contains 6.02 x 1023 particles (Avogadro’s number), we can interpret the following equation: H2 + I2 …
How to calculate number of moles and molecules in a sample of molecule
In this video, you learn how to calculate the number of moles and molecules of a sample of molecue To learn how to calculate moles and atoms of an element click here. …
Continue Reading about How to calculate number of moles and molecules in a sample of molecule →
How to calculate the number of moles and atoms from a sample of an element
In the above video, you learn how to calculate the number of atoms in 20.0g of Calcium. If you want to learn more about the mole concept, click here …
Continue Reading about How to calculate the number of moles and atoms from a sample of an element →
Why do chemists need the Mole concept?
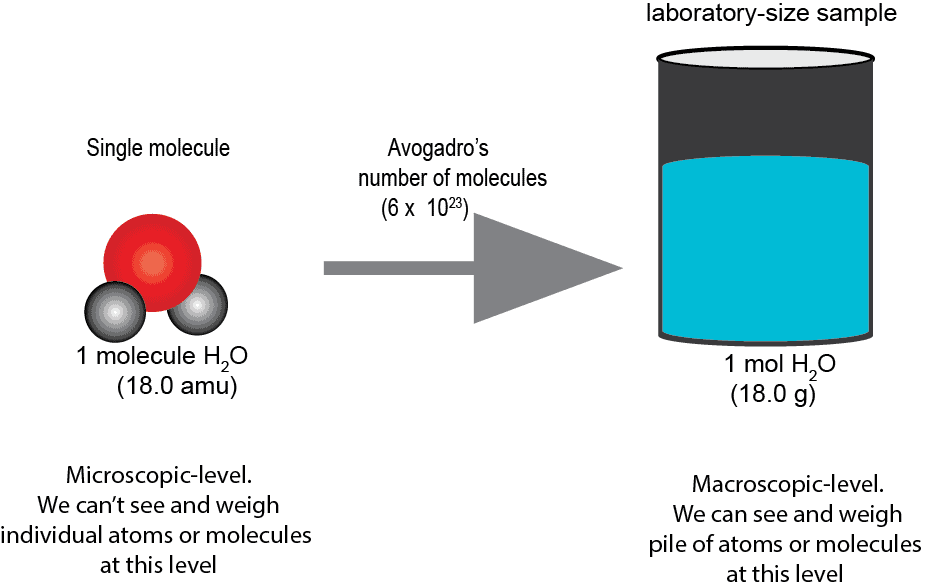
Why do chemists need the Mole Concept? Chemists need the mole concept to bridge the gap between the microscopic world of atoms to the macroscopic world of humans. As you know, the molecular level …
Continue Reading about Why do chemists need the Mole concept? →