Why does the volume of gas increase as the temperature increase?
As temperature of gas molecules increase, they become more energetic, so they move a lot faster and spread out a lot more to occupy more space. Let’s use the model below to explain.
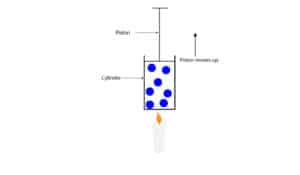
The model consists of gas molecules in a metal cylinder with a piston that can move up or down to keep the pressure inside the cylinder equal to the pressure outside. The piston has a seal around its edges so that no air can get in or out of the cylinder.
How do we know that the outside pressure is constant?
Since the outside pressure is the same as the atmospheric pressure, we can assume that the pressure outside the cylinder stays the same (constant).
What will happen when we put a burning candle under the cylinder?
If we put a burning candle under the cylinder, it will transfer its energy to the gas molecules inside the cylinder, increasing their kinetic energy. As these gas molecules become more energetic, they will bounce off the walls of the cylinder and piston, moving the piston up. As the piston moves up, the space (volume) occupied by the gas molecules inside the cylinder increases.
In short, we say that as temperature increase, the volume occupied by the gas molecules increase as well.
How can we use mathematics to describe the relationship between volume and temperature?
Mathematically, we can express the relationship as: V ∞ T.
This expression is read as: volume is directly proportional to temperature. Directly proportional is a fancy way of saying that as the temperature increase, the volume of gas increase in a precise way.
We can remove the proportionality sign and introduce an equal sign and a proportionality constant, K. If we do, we can rewrite the expression as V = KT.
If we divide both sides of the expression by T, we will get: V/T =K.
This expression simply says that if we divide the volume of a gas by its temperature, we will get a constant, K. Since Charles was the first to uncover this relationship, it’s usually called the Charles law.
Why does the volume of gas decrease as the temperature decrease?
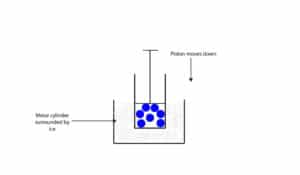
As temperature of gas molecules decrease, they become less energetic and move a lot slower and spread out a lot less. Thus, as temperature decrease, the volume of the gas decrease as well. Let’s use the model below to explain.
The model is the same as the one above, but surrounded by ice. Since the ice is at a lower temperature, energy will be transferred from the cylinder and gas molecules to the ice. This energy transfer causes the gas molecules to move a lot less, decreasing the amount of space they occupy and causing the piston to drop.
To learn more about why the volume of gas can’t be zero, click here.
To learn more about the general properties of gases, click here.