Empirical formula expresses the simplest mole ratio of the elements in a compound or molecule. To determine empirical formula from percent composition, you must first convert the percentage composition values to masses. You do this conversion by assuming that you have 100 g of your compound. Keep in mind that this 100.00 g is just a definition. Next, you then multiply the percentage composition value by the 100.00 g. The answer you get will be how much mass the element contributes to the mass of the compound. Keep in mind that you must divide each composition by 100 before you multiply. Now, let’s use the following example to determine the empirical formula from percentage composition.
Let’s say you carried out an elemental analysis of two compounds that consist of iron and oxygen. From the analysis, you found that one of the compounds consists of 77.73% of Fe, while the other 69.94% of Fe. From the data, determine the empirical formulas of the compounds?
Solution
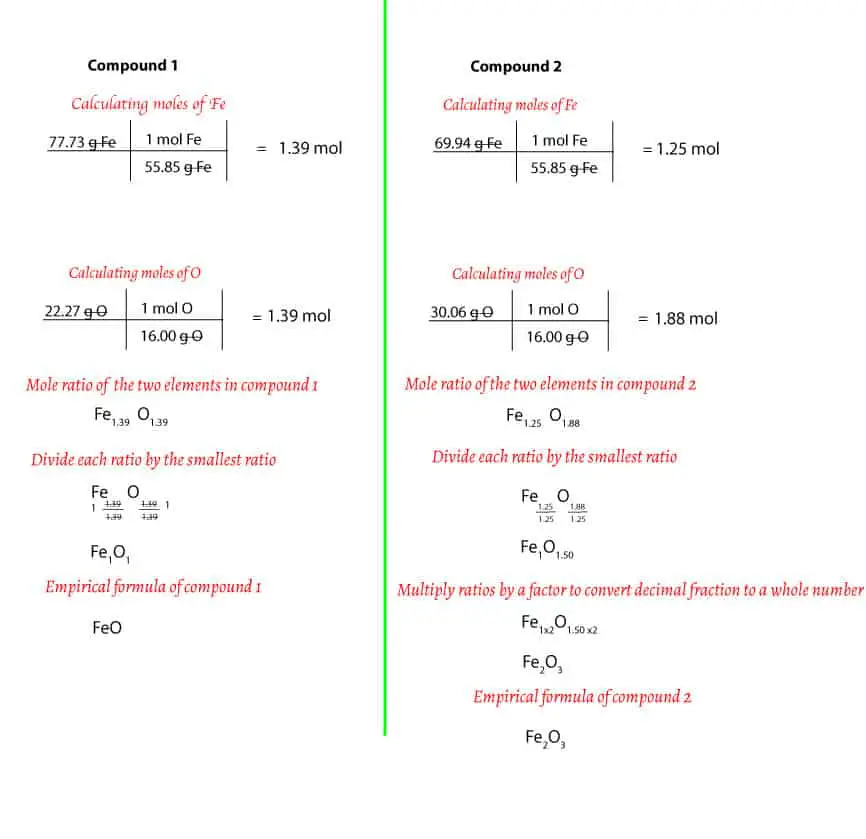
Step 1: calculating the mass of each element in grams
For compound 1, we must assume that we have 100 g of it. Next, you must find the mass of each element in the 100 g of the compound. To do this, you will multiply the percentage composition by the 100 g. If you do, you will get:
77.73/100 * 100 g = 77.73 g Fe
Now, since each compound consists of iron and oxygen, then it follows that we can get the mass of oxygen by subtracting the mass of Fe from the mass of the compound (100 g). This is because if you add the mass of oxygen to the mass of iron, you will get the total mass of the compound (law of conservation of mass)
For compound 1, we will do: 100 g – 77.73 g Fe = 22.27 g O. If you are following, you will notice that compound 1 consists of 77.73 g of Fe and 22.27 g of O.
Similarly, for compound 2, we can find the mass of Fe by multiplying the percentage composition by the 100 g. If you do, you will get:
69.94/100 * 100 g = 69.94 g Fe
Also, you can find the mass of O by subtracting the mass of Fe from the mass of the compound. If you do, you will get:
100 g – 69.94 g Fe = 30.06 g O
After calculating, you will notice that compound 2 consist of 69.94 g of Fe and 30.06 g of O.
Step 2: converting the mass of each element to moles
Now that we know the mass of each element, the next thing is to calculate the moles of each element. How do we do this? To calculate moles, first, you must use the periodic table to determine the molar mass of Fe and O. If you do, you will notice that Fe has a molar mass of 55.85 g/mol, while O has a molar mass of 16.00 g/mol. Second, you must use this molar mass as a conversion factor to convert from grams of each element to moles of each element. For compound 1 and 2, the conversions will look like this:
For compound 1,
Moles of Fe: 77.7 g x 1 mol/55.85 g = 1.39 mol Fe
Moles of O: 22.27 g x 1 mol/16.00 g = 1.39 mol O
For compound 2,
Moles of Fe: 69.94 g x 1 mol/55.85 g = 1.25 mol
Moles of O: 30.06 g x 1 mol/16.00g = 1.88 mol O
Step 3: divide the mole ratios by the simplest ratio
For compound 1, the mole ratio of Fe to O are 1.39 to 1.39 respectively. If you divide each ratio by 1.39, you get 1. Therefore, the mole ratio of Fe and O is 1 to 1. If we take this ratio into account, then the empirical formula of compound 1 is FeO
For compound 2, the mole ratio of Fe to O are 1.25 to 1.88 respectively. If you divide each ratio by the lowest, 1.25, you will get 1 and 1.5 respectively. Therefore, the mole ratio of Fe and O is 1: 1.5. However, if we take this ratio into account, then the empirical formula of compound 2 is Fe1O1.5. But as you may recall, atoms do not exist as fractions. Because of this, we must convert 1.5 to the nearest whole number. To do this, we must multiply both ratios by 2. If we do, we will get 2 for Fe and 3 for O. Therefore, the empirical formula of compound 2 is: Fe2O3.
Here is a summary of the above calculations:
How to determine empirical formula from molecular formula
Empirical formula expresses the simplest mole ratio of the elements in a compound or molecule. While molecular formula expresses the actual number of each element in a molecule. As you can tell, empirical and molecular formula are related, but not identical. Usually, you can derive the molecular formula from the empirical formula by multiplying the mole ratio in the empirical formula by a specific number. In the same way, you can derive the empirical formula from the molecular formula by dividing the mole ratio in the molecular formula by a specific number. Let’s use the following example to illustrate.
For instance,
if the molecular formula of an organic molecule is C2H4O2, what is its empirical formula?
Solution
Since the molecular formula is C2H4O2,it follows that the mole ratios of carbon, hydrogen, and oxygen in the formula are 2:4:2 respectively. From these ratios, you can see that each of them can be divided by 2 without leaving a remainder. Now, since empirical formula expresses the simplest ratio, then it follows that we can divide the ratios by 2 to get the empirical formula. If we do, we will get:
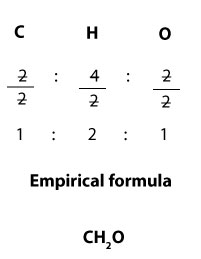
Since 1:2:1 is the simplest ratio, we can write the empirical formula as C1H2O1, which is the same as CH2O. We can get back the molecular formula—C2H4O2— by multiplying the mole ratios in the empirical formula— C1H2O1— by 2.
Generally, the relationship between empirical and molecular formula can be expressed as:
- (Empirical formula)n = molecular formula
Where n can be 1,2,3, and so on
In other words, the above relationship is the same thing as saying how many empirical formulas (n) can go into making the actual compound. Therefore, another way you can find n is if you know the molar mass of the actual compound and the molar mass of the empirical formula. If you do, you can divide the molar mass of the actual compound by the molar mass of the empirical formula to get the subscript, n. Once you get n, you will then multiply the subscripts in the empirical formula by n to get the molecular formula.
If you want to learn how to determine empirical formula from combustion analysis, click here.