You may have noticed that when you connect your toaster to electricity, as the wire in the toaster gets heated, it glows red as it emits red light, and as its temperature continues to rise, it glows orange as it emits orange light, and as it gets really hot, it glows white as it emits all wavelengths of visible light all at once. From these observations, we can say that when an object is heated, the radiation it emits changes as its temperature rises.
So, what causes an object to emit radiation when heated?
To answer that, a scientist called Max Planck, suggested that when an object is heated, the atoms of that object absorb and emit radiation in small packets. And that these small packets hold a minimum amount of energy. Just as a molecule is the smallest particle (packet) of a compound and holds the chemical properties of the compound. Max Planck called this packet of energy quantum. As an analogy to this quantum concept, let’s consider an illustration of a ball rolling down a ramp and a ball rolling down stairs:
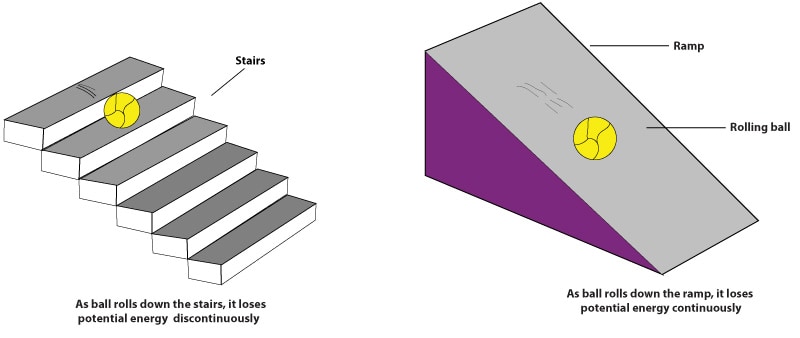
As you can see, as the ball rolls down the ramp, it loses potential energy continuously, but as it rolls down the stairs, it loses potential energy in packets or bundles (discontinuously). So too do heated objects emit radiation in packets.
Planck further proposed that the energy of a quantum is directly proportional to the frequency of the radiation. Mathematically, we can express this relationship as:

As you can see from equation (1), when you multiply Planck’s constant by the frequency of any light, you will get the energy for one quantum of that light. For instance, if you multiply Planck’s constant by the frequency of red light, you will get the energy for one quantum of red light.
In the same way, from equation (2), you will notice that energy is inversely proportional to the wavelength. This means that as the wavelength decreases, the energy of light increases. Similarly, as the wavelength increases, the energy of light decreases.
By doing such comparisons and calculations, Planck was able to predict the spectra of heated objects that agreed well with those from experiments. Because of this, Planck’s work gave birth to a new theory of physics called the Quantum Theory. Unlike classical physics, quantum theory is useful in our understanding of how energy interacts with matter at the atomic and subatomic level.
To learn how Bohr applied the quantum concept to enhance our understanding of the atomic theory, Click here.