Exceptions to the octet rule include the following:
Some atoms have fewer than eight valence electrons
One exception to the octet rule is that in the real world some atoms in molecules or compounds do have fewer than eight (octet) valence electrons. Some examples of these include:
- Hydrogen (H) in H2. In hydrogen molecule (H2), the maximum number of electrons a hydrogen atom can have is two. Recall that from quantum mechanics, hydrogen can hold a maximum of only two electrons in its 1s-orbital.
- Beryllium (Be) in BeF2. In compounds of Beryllium, such as BeF2 experiments show that Be forms two single bonds with fluorine. Here is the structure showing the single bonds:
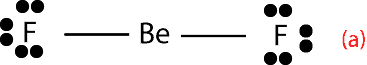
As you can see, from the structure above, Be only has 4 valence electrons.
However, Lewis rules, predict that Beryllium should rather form double bonds in order to have an octet. Here are the double bonds:
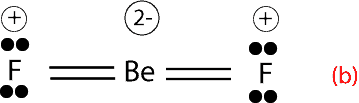
If you calculate the formal charge for each atom, you will notice that fluorine, which is the most electronegative element has a positive formal charge. Having a positive formal charge on the most electronegative atom, makes the compound unstable, and because of this instability, structure (b) is not observed in experiments.
- Boron (B) in BCl3. In compounds of Boron, such as BCl3 experiments show that B forms three single bonds with chlorine. Here are the single bonds:
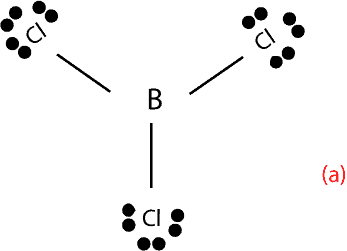
As you can see, from the structure above, B has only 6 valence electrons.
However, Lewis rules, predict that one of the bonds between Boron and chlorine should be a double bond in order for B to have an octet. Here are the bonds predicted by Lewis rules:
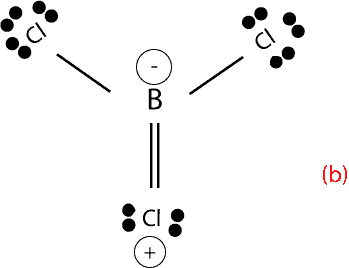
Again, if you calculate the formal charge for each atom, you will notice that chlorine, which is the most electronegative element has a positive formal charge. Having a positive formal charge on the most electronegative atom, makes the compound unstable, and because of this instability, structure (b) is not observed in experiments.
Some atoms have more than eight valence electrons
Another exception to the octet rule is that the central atoms of certain molecules may have more than eight (octet) valence electrons (expanded octet). This usually happens when the central atom has more than four atoms bonded to it. Examples of such atoms include phosphorus in the molecule PCl5 (phosphorus pentachloride) and sulfur in the molecule SF6 (sulfur hexafluoride). These atoms which are in the third row (period 3) of the periodic table usually include their empty d-orbitals in their valence shell, and these d-orbitals also take part in bonding.
Some atoms have an odd number of valence electrons
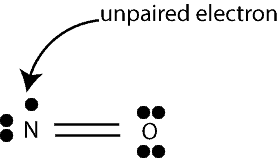
The third exception is that some molecules have an odd number of valence electrons. Examples of such molecules include NO2 NO, and CH3. If we draw the Lewis structures of such molecules, we will notice that each molecule has one unpaired electron. Such molecules, sometimes called free radicals are highly reactive and will easily combine with other atoms to form molecules. Here is the Lewis structure of NO showing the unpaired electron:
If you want to learn the rules on how to write Lewis structures, click here