To make sense of these properties, we must recall that molecular compounds consists of independent units called molecules. These molecules are formed when two or more atoms combine by sharing electrons. While ionic compounds consist of positive and negative ions held together in a repeating three-dimensional network called crystal lattice. In the crystal lattice, the simplest grouping of the positive and negative ions is called the formula unit. Unlike molecules, formula units do not exist by themselves outside the crystal.
Now, let’s take a look at the following particle model showing a two-dimensional view of sodium (positive) and chloride (negative) ions held together in a crystal of sodium chloride
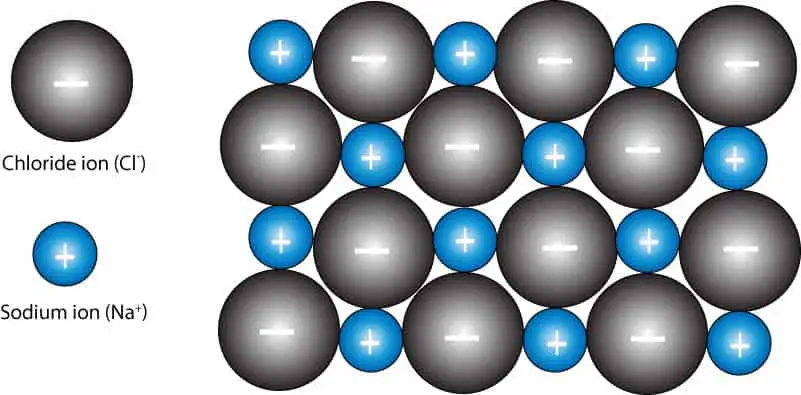
As you can see, the sodium and chloride ions are tightly held by an attractive force as a result of the positive charge on sodium and negative charge on chloride.
Again, let’s take a look at the following particle model showing a container filled with oxygen molecules
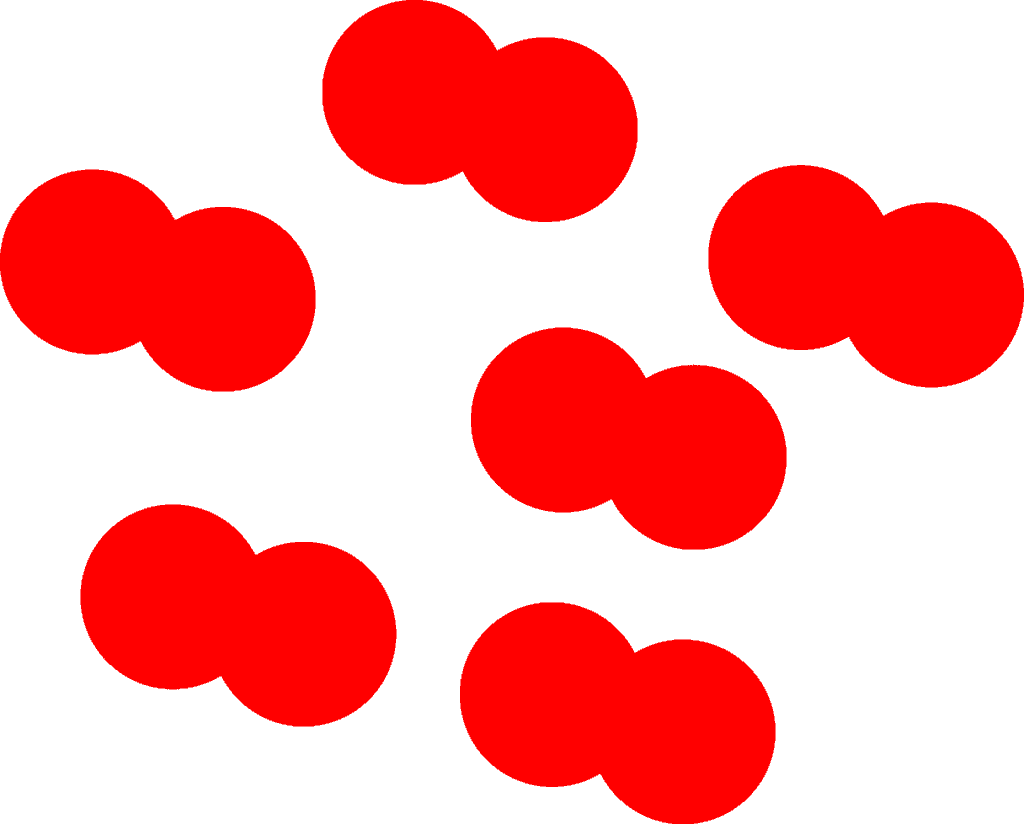
As you can see, the oxygen molecules are independent of each other and therefore can exist individually.
As a result of how molecular compounds form, their general properties include the following:
- form when nonmetals react with other nonmetals
- exist as solids, liquids, or gases
- soft and brittle
- poor conductors of heat and electricity, because they hold their electrons so tightly
- low melting and boiling points, because the attractive forces between molecules (intermolecular forces) are generally weak
- Many are insoluble in water but soluble in organic solvents. Insoluble in water because of dissimilar polarity but soluble in organic solvents because of similar polarity. Similar polarity with organic solvents allow molecular compounds to interact well with organic solvents
Examples of molecular compounds include H2O, CO2 and many others.
As a result of how ionic compounds form, their general properties include the following:
- form when metals react with nonmetals
- exist as crystalline solids
- hard and brittle
- high melting points, because the attractive forces between ions (intramolecular forces) are stronger
- high boiling points, because the attractive forces between ions are stronger
- good conductors of electricity when molten or dissolved in water, because under these conditions the ions can move about, allowing electrons to flow through
- poor conductors of heat and electricity when solid, because under this condition, the ions can’t move about, restricting the flow of electrons
- Many are soluble in water, because they share similar polarity. This similar polarity allow some ionic compounds to interact well with water
Examples of ionic compounds include NaCl, CaSO4 and many others.