Here is our problem:
Sulfuric acid (H2SO4) reacts with sodium hydroxide (NaOH) according to the equation:
NaOH(aq) + H2SO4(aq) —- > Na2SO4(aq) + H2O(l)
If 25.0 mL of H2SO4 solution requires 29.3 mL of 0.250 M NaOH to neutralize it, calculate the concentration of H2SO4
To start, we must check to ensure the chemical equation is balanced. From the equation, you will notice there are two atoms of sodium (Na) on the right-hand side of the equation and one atom of sodium on the left-hand side of the equation. Also, there are three atoms of hydrogen on the left-hand side of the equation and two atoms of hydrogen on the right-hand side of the equation. Clearly, the equation is not balanced. To balance it, we will put 2 in front of NaOH and 2 in front of H2O. Once we do, our equation will appear as:
2NaOH(aq) + H2SO4(aq) —- > Na2SO4(aq) + 2H2O(l)
Now that we have a balanced equation, here is what we know from question
Volume of of H2SO4 = 25.0 mL
Volume of NaOH = 29.3 mL
Molarity of NaOH = 0.250 M
From the above information, you can tell that we know everything about NaOH. That is, we know its volume and concentration and from this information we can calculate the moles of NaOH. Therefore, indirectly, the question is asking us to first find the moles of H2SO4, and then its Molarity by using the moles of NaOH.
And here are the steps we need to follow to easily solve the question

As you recall, Molarity is a unit for concentration, and it is calculated by dividing moles of solute by volume of solution in liters. Because of this, Molarity has units of moles per liter (mol/L), and often shortened in chemistry as uppercase, M. Therefore, the first step in the diagram is saying when given volume in milliliters (mL) you must immediately convert to Liters (L). Once you convert your volume to Liters, you immediately multiply volume in liters by the the Molarity to convert to moles. Why do you have to convert to moles? You do because the chemical equation speaks in the language of moles.
From our question, we know both the volume and Molarity of NaOH, this means that we know everything about NaOH and can use its volume and Molarity to calculate its moles. Once we calculate the moles of NaOH, we will then use the mole-ratio (coefficients) from the balanced chemical equation to convert from moles of NaOH to moles of H2SO4. This step is illustrated in the diagram as moles of solution A to moles of solution B.
Once you get moles of solution B, you will divide moles of solution B by its corresponding volume in Liters as provided in the question. Let me stress, volume of solution B in liters. If volume of solution B is not in liters, you must convert it to liters before you divide.
Here is a summary of our calculation base on the steps outlined above.
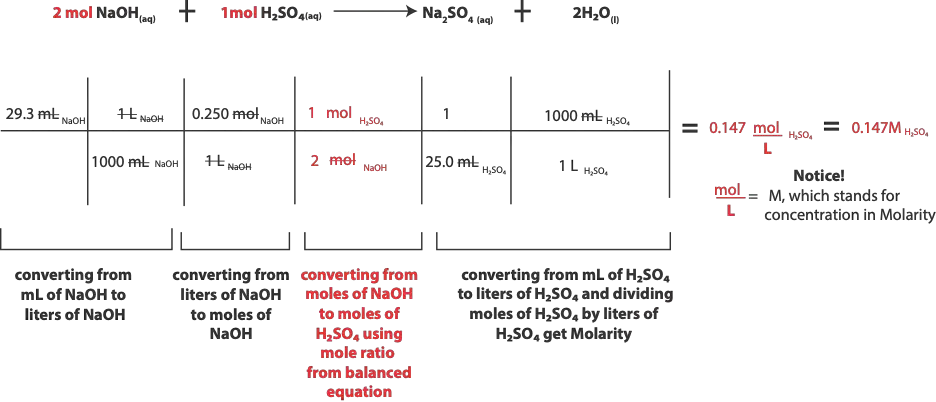
As you might have realized, an important part of the calculation is to recognize that Molarity (M) is a derived unit, and it’s the same as writing (mol/L). Therefore 0.250 M is the same as writing 0.250 mol/L.
And writing it as mol/L will allow us cancel out Liters and leave out moles of NaOH, which is exactly what we want so that we can use the mole-ratio from the balanced chemical equation to convert from moles of NaOH to moles of H2SO4.
You can solve similar solution stoichiometry problems by applying the above steps in addition to the steps outlined in the following diagram
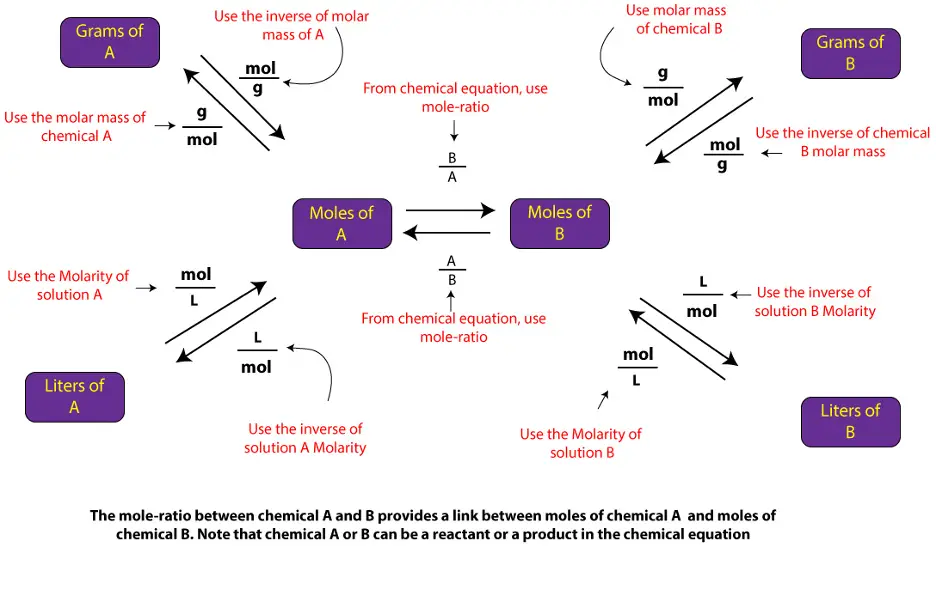
To calculate the volume of one chemical from grams, click here
To calculate solution concentration in Molarity, click here.