Vapor pressure is the pressure exerted by the vapor above a liquid in a closed container when the rate of evaporation of the liquid is equal to the rate of condensation of its vapor. However, vapor pressure lowering occurs when the vapor of pure liquid is higher than the vapor pressure of its solution when a non-volatile solute dissolves in it. This lowering occurs because the solute particles disrupt the attractive forces between the solvent molecules, making it harder for solvent molecules to leave and return to the liquid phase. For example, the vapor pressure of pure water is higher than that of its solution — salt water. Because in salt-water sodium ions (Na+) and chloride ions (Cl–) disrupt the attractive forces between water molecules.
Generally, the vapor pressure of pure solvents will decrease when non-volatile solute is added to the solvents. However, the amount of this decrease will depend on the solute concentration, which we can calculate by applying Raoult’s law. Raoult’s law is expressed as:
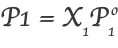
where,
P1 is the vapor pressure of the pure solvent at the same temperature
P1 is the vapor pressure of the solvent over the solution
X1 is the mole fraction of solvent in the solution
Now, let’s apply Raoult’s law to solve the following problem
Ethylene glycol, HOCH2CH2OH, is used as an antifreeze. What is the vapor pressure of water above the solution of 100.0 mL ethylene glycol and 100.0 mL water at 90 ºC.
Densities of ethylene glycol is 1.15 g/mL and water 1.00 g/mL, and vapor pressure of water at 90 ºC is 525.8 mm Hg.
To solve this problem using Raoult’s law, we must first calculate moles of ethylene glycol and moles of water so that we can calculate mole fraction of water. However, to calculate moles, we must use the densities provided to convert volume of ethylene glycol and volume of water to grams. Once we get grams, will use molar mass of each chemical to convert from grams to moles for that chemical. Here are the calculations:
For moles of ethylene glycol, will do:
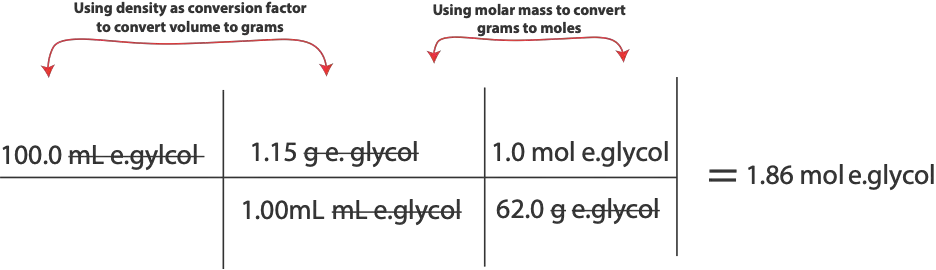
For moles of water, will do:
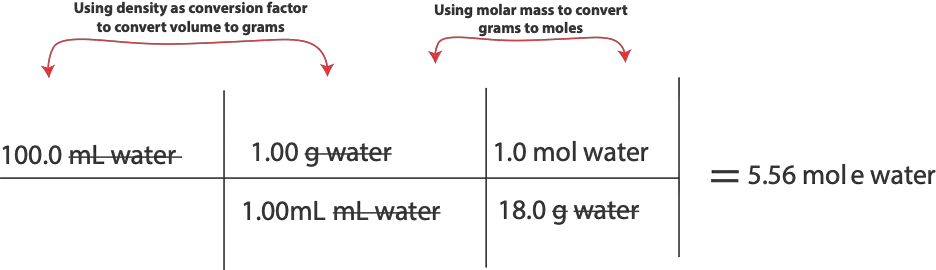
To calculate mole fraction of water, will do,

Applying Raoult’s law, the calculation is:

And the vapor pressure of water above the solution is 394 mm Hg
One important application of colligative properties is in the petroleum refinery, where petroleum is fractionally distilled and separated according to boiling points.
To learn about boiling point elevation click here, for freezing depression click here, for colligative properties definition, click here.