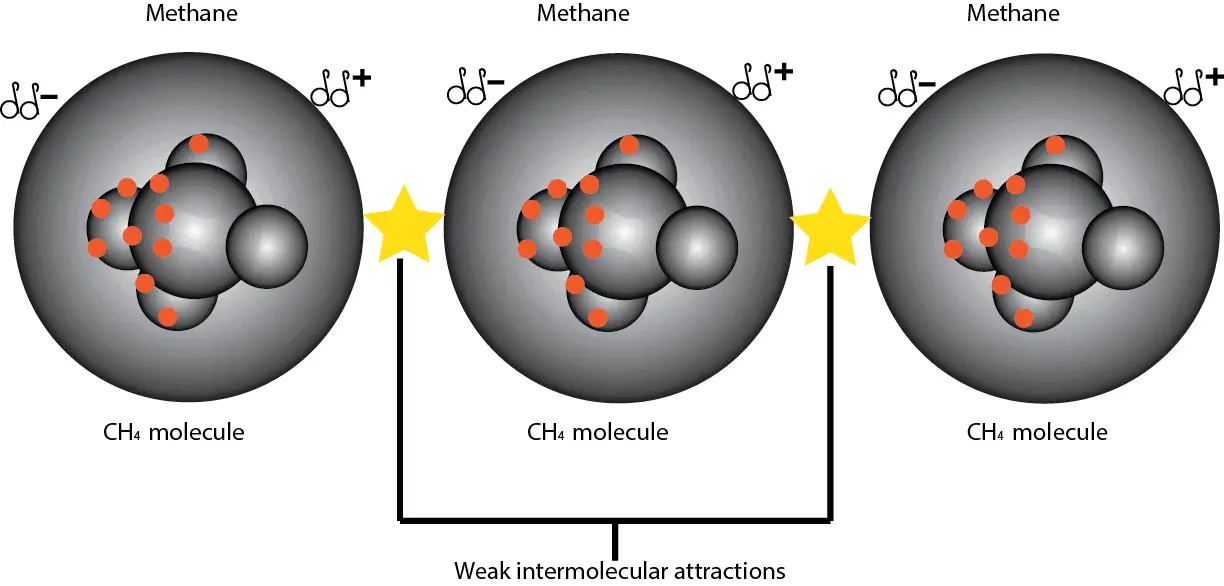
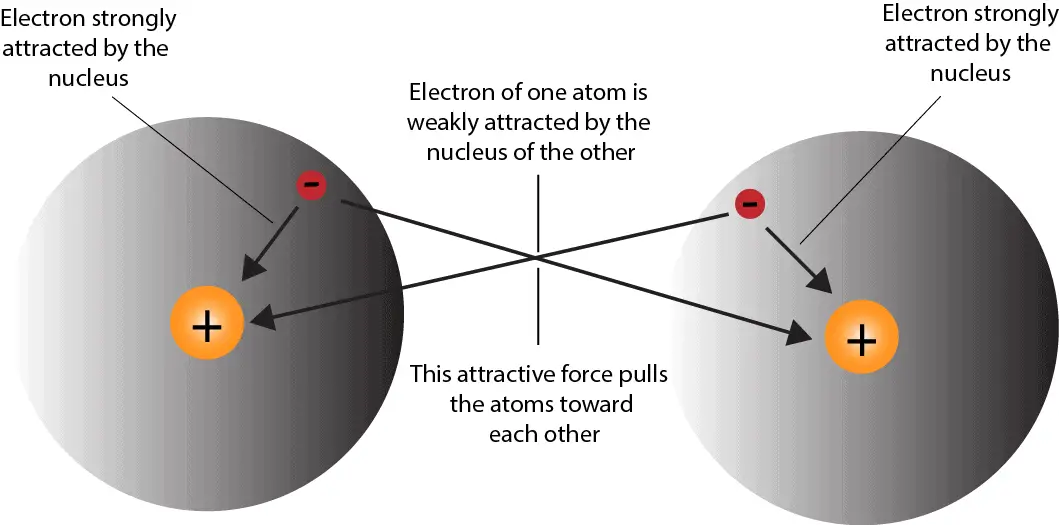
What’re Vander Waals forces? Vander Waals forces are attractive forces between nonpolar molecules. How’s this force produced? As electrons constantly move at random in a molecule, there comes a time …
How do atoms form an ionic bond?
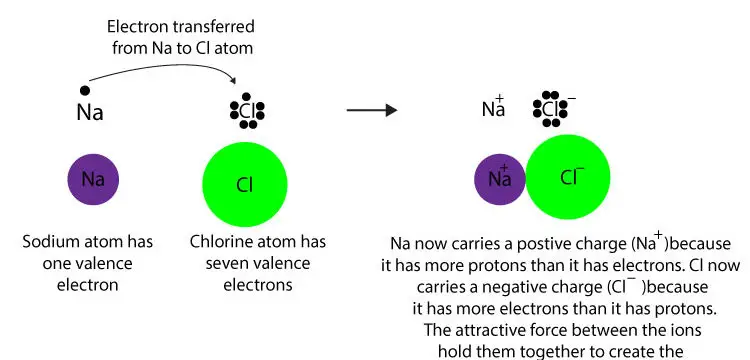
What’s an ionic bond? An ionic bond is a force of attraction between a positive and a negative charged ion. These oppositely charged ions are usually produced when a metal transfers its electron to a …
How do atoms form covalent bond?
How do atoms form covalent bond? Atoms form covalent bonds when two or more nonmetals share valence electrons. What’s a covalent bond? A covalent bond is a force of attraction between two or more …
What’s a chemical bond? And what’re intermolecular and intramolecular forces?
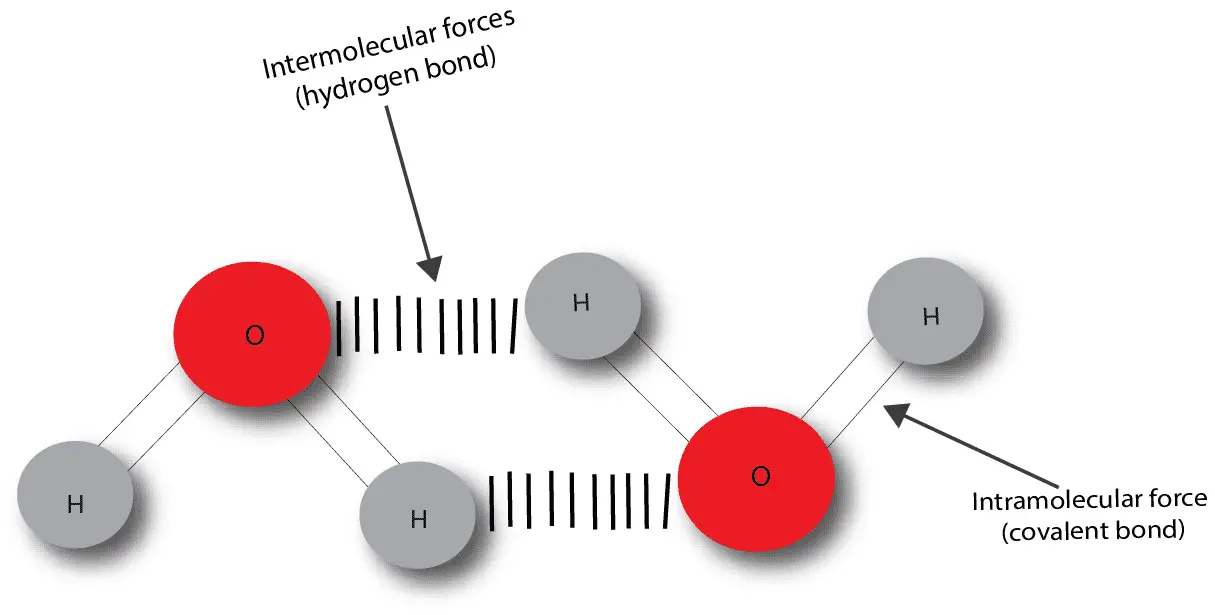
What’s a chemical bond? A chemical bond is an attractive force between two or more atoms. This attractive force can be intermolecular or intramolecular. The intermolecular forces are weaker and exists …
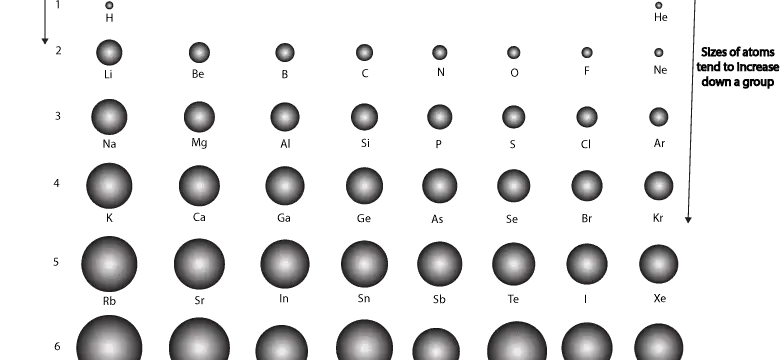
How does atomic size vary across and down the periodic table?
How does atomic size vary across and down the periodic table for main group or representative elements? For main group elements, atomic size gets larger as you go down a group (column) and atomic size …
Continue Reading about How does atomic size vary across and down the periodic table? →
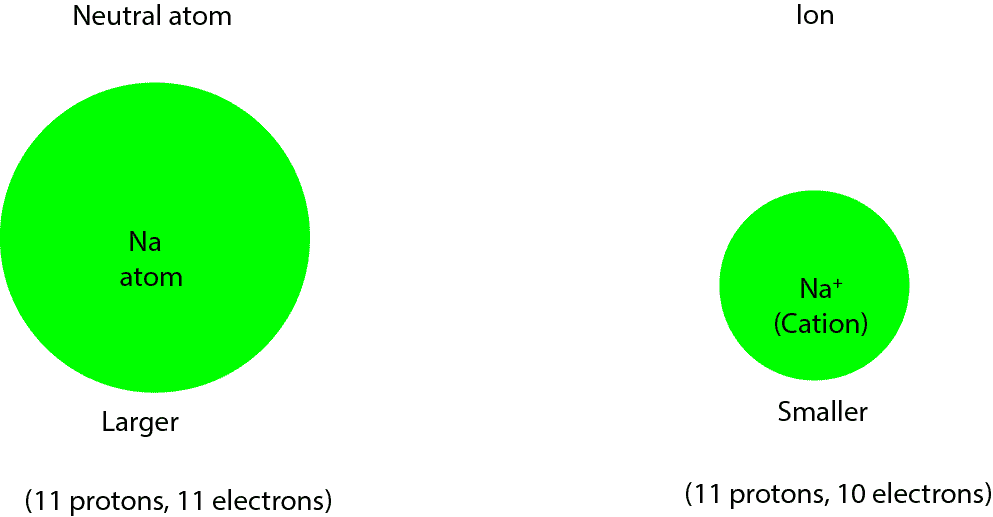
Can a cation or anion be larger than its neutral atom?
Can a cation (positively charged ion) be larger than its neutral atom? No! A cation is usually smaller than its neutral atom. Let’s explain this further with an example. Given neutral sodium atom …
Continue Reading about Can a cation or anion be larger than its neutral atom? →