Molality (m) is the moles of solute dissolved in kg of solvent. While Molarity(M) is the moles of solute dissolved in volume of solution in …
How to solve solution stoichiometry step-by-step to determine the volume of a chemical when given only its Molarity and grams of another
Here is our problem: Zinc reacts with HCl to produce H2 gas according to the equation: Zn(s) + HCl(aq) ---> ZnCl2(aq) + H2(g) If you have 10.0 g of Zn metal, …
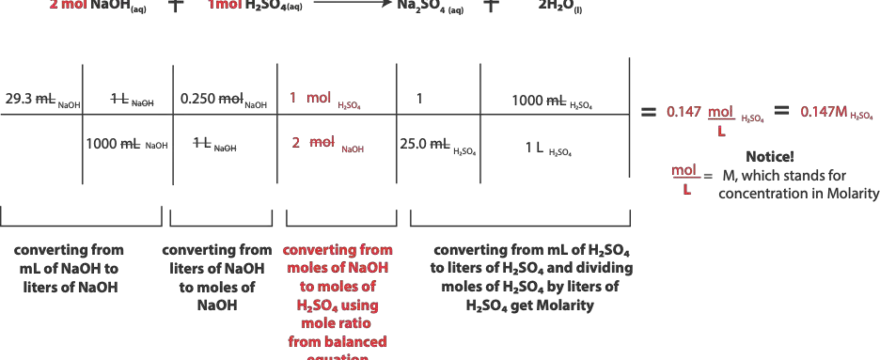
How to solve solution stoichiometry step-by-step to determine the Molarity of a chemical when only its volume and molarity and volume of another are known in the balanced chemical equation
Here is our problem: Sulfuric acid (H2SO4) reacts with sodium hydroxide (NaOH) according to the …
How to calculate theoretical and percentage yield
How to use limiting reactant to determine theoretical yield Since the limiting reactant is completely used up first during a reaction, it follows that the limiting reactant determines how much of …
Continue Reading about How to calculate theoretical and percentage yield →
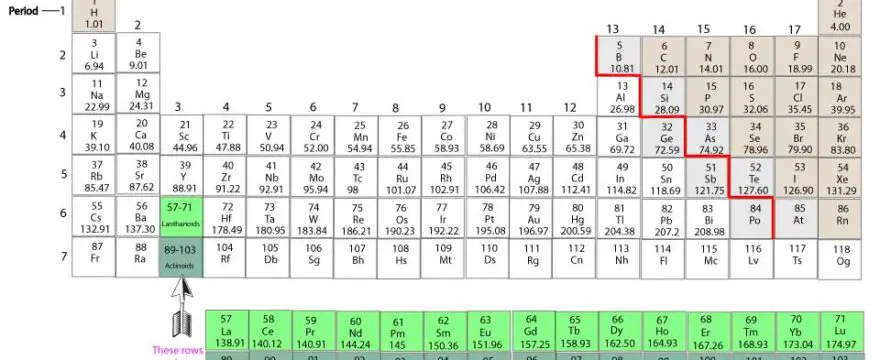
How to use the periodic table to determine an element’s valence electrons
Valence electrons are the electrons in the outermost shell of an atom. These electrons are easily accessible and used by atoms to bond with one another. These bonds usually form when atoms lose, gain, …
Continue Reading about How to use the periodic table to determine an element’s valence electrons →
How to calculate solution concentration in Molarity
What’s Molarity? Molarity, with symbol M, is defined as the number of moles of solute present in a liter of solution. Mathematically, Molarity can be expressed as: Molarity, M = moles of …
Continue Reading about How to calculate solution concentration in Molarity →