Osmosis is the movement of solvent particles through a semipermeable membrane from a region of higher solvent concentration (lower solute concentration) to a region of lower solvent concentration …
What are colligative properties and what are the effects of electrolytes on these properties?
Colligative properties of solutions are properties that depend only on the concentration of solute particles (ions or molecules) dissolved in the solution. Colligative properties include: Vapor …
What ‘re the colligative properties boiling point elevation and freezing point depression, and how do you calculate them?
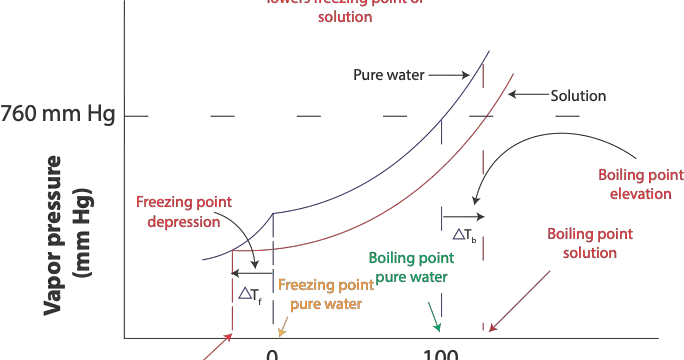
When the vapor pressure of pure water is equal to standard atmospheric pressure (1 atm or 760 mm Hg) at 100 ºC pure water will boil at 100 ºC, which is its normal boiling point. However, since the …
What is colligative property vapor pressure lowering and how do you calculate it using Raoult’s law?
Vapor pressure is the pressure exerted by the vapor above a liquid in a closed container when the rate of evaporation of the liquid is equal to the rate of condensation of its vapor. However, vapor …