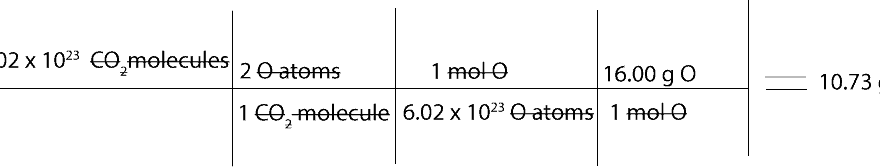Here is our problem: Zinc reacts with HCl to produce H2 gas according to the equation: Zn(s) + HCl(aq) ---> ZnCl2(aq) + H2(g) If you have 10.0 g of Zn metal, …
How to interpret a chemical equation in terms of moles to write mole-mole ratio
For example, when hydrogen (H2) reacts with iodine (I2) to produce hydrogen iodide (HI), we can write a balanced chemical equation for the reaction as: H2 + I2 --> 2HI. Now, let's …
How to interpret and use chemical formula to go from molecules of one substance to grams of another
To use a chemical formula to go from molecules to atoms or grams, we must first learn how to interpret the chemical formula in terms molecules and atoms. Once we do, we can then proceed to write ratio …
How to determine limiting reactant
A limiting reactant is a chemical in short supply during a chemical reaction. Usually, we can run a chemical reaction in one of two ways. The first is: using the exact mole amounts (stoichiometric) …
Using a chemical equation to convert from moles of one chemical to moles of another in stoichiometry
Mole to mole stoichiometry Use the following equation: 2Fe + 3S ----> …