The general rules for writing Lewis structures include the following:
Rule 1
Determine the total number of valence electrons in the molecule. To do this, you will need the periodic table. And recall that for main group elements the valence electrons can be determined from the group number. For instance, for the first two columns in the periodic table number 1 and 2, the valence electrons of elements in these columns is the same as the group number. For the last six columns numbered (13 – 18), the valence electrons is the same as the last digit of the group number. That is elements in group 13 will have 3 valence electrons, and elements in group 18 will have 8, except for He which has 2.
If the molecule is a positive ion (molecular cation), subtract electrons from the total number of valence electrons. If the molecule is a negative ion (molecular anion), add electrons to the total number of valence electrons.
Why do you have to add or subtract electrons when it’s a molecular ion?
Recall that to make a positive ion, an atom must lose electrons, and to make a negative ion, an atom must gain electrons. Therefore, gaining electrons increases the total number of electrons in a negative ion, while losing electrons decreases the total number of electrons in a positive ion. Here is an example: in NH4+ (ammonium ion), nitrogen (N) has 5 valence electrons, while H has 1 valence electron. But since there are four atoms of hydrogen in NH4+ it follows that we must multiply the valence electrons of H by 4. If we do, then the total number of valence electrons in NH4+ will be: 5 + 4(1) = 9. But wait, NH4+ has a 1+ positive charge. This means that we must subtract 1 from the total number of valence electrons. If we do, then NH4+ will be left with a total of 8 valence electrons.
Similarly, for the sulfate ion, SO42-, with a charge of 2-, we must add 2 electrons to the total number of valence electrons. That is 6 + 4(6) + 2 = 32 valence electrons.
Rule 2
Draw the bond skeleton of the molecule. To do this, we must first identify the central atom. In most molecules, the central atom is usually the atom written first in the chemical formula of the molecule. However, there are exceptions to this rule. For instance, in H2O, hydrogen is the first atom in the formula, but it’s not the central atom. This is because hydrogen usually forms one bond. When dealing with molecules like water, we must ask ourselves can the first atom form more than one bond. If no, then, we move on to the next atom and ask the same question. We keep moving and asking until we identify the central atom. If we do for H2O, we will notice that oxygen is the central atom. If so, the bond skeleton will appear as:
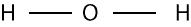
But notice that the bond (dashed line) consists of two electrons.
Rule 3
Distribute the remaining electrons to atoms attached to the central atom until each gets eight electrons (excluding hydrogen). If there is a surplus of electrons after every atom gets eight, give the rest to the central atom. If electrons run out before each gets eight, use the lone electron pairs from one atom to form multiple bonds with another atom. For H2O, there are a total of 8 valence electrons, but H can only have a duet (two) of electrons. As a result, the Lewis structure will appear as:
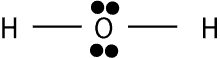
If you want to learn more about the exceptions to the octet rule, click here.